Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells
- PMID: 36926196
- PMCID: PMC10011497
- DOI: 10.3389/fphys.2023.1149063
Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells
Abstract
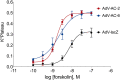
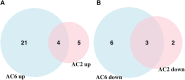
Human airway smooth muscle (HASM) is the primary target of ßAR agonists used to control airway hypercontractility in asthma and chronic obstructive pulmonary disease (COPD). ßAR agonists induce the production of cAMP by adenylyl cyclases (ACs), activate PKA and cause bronchodilation. Several other G-protein coupled receptors (GPCR) expressed in human airway smooth muscle cells transduce extracellular signals through cAMP but these receptors elicit different cellular responses. Some G-protein coupled receptors couple to distinct adenylyl cyclases isoforms with different localization, partly explaining this compartmentation, but little is known about the downstream networks that result. We used quantitative phosphoproteomics to define the downstream signaling networks emanating from cAMP produced by two adenylyl cyclases isoforms with contrasting localization in uman airway smooth muscle. After a short stimulus of adenylyl cyclases activity using forskolin, phosphopeptides were analyzed by LC-MS/MS and differences between cells overexpressing AC2 (localized in non-raft membranes) or AC6 (localized in lipid raft membranes) were compared to control human airway smooth muscle. The degree of AC2 and AC6 overexpression was titrated to generate roughly equal forskolin-stimulated cAMP production. 14 Differentially phosphorylated proteins (DPPs) resulted from AC2 activity and 34 differentially phosphorylated proteins resulted from AC6 activity. Analysis of these hits with the STRING protein interaction tool showed that AC2 signaling is more associated with modifications in RNA/DNA binding proteins and microtubule/spindle body proteins while AC6 signaling is associated with proteins regulating autophagy, calcium-calmodulin (Ca2+/CaM) signaling, Rho GTPases and cytoskeletal regulation. One protein, OFD1, was regulated in opposite directions, with serine 899 phosphorylation increased in the AC6 condition 1.5-fold but decreased to 0.46-fold by AC2. In conclusion, quantitative phosphoproteomics is a powerful tool for deciphering the complex signaling networks resulting from discreet signaling events that occur in cAMP compartments. Our data show key differences in the cAMP pools generated from AC2 and AC6 activity and imply that distinct cellular responses are regulated by these two compartments.
Keywords: adenylyl cyclase; cAMP; g-protein coupled receptors; human airway smooth muscle; phosphoproteomics.
Copyright © 2023 Cattani-Cavalieri, Li, Margolis, Bogard, Roosan and Ostrom.
Conflict of interest statement
AB was employed by AB Research LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Adenylyl cyclase 2 selectively couples to E prostanoid type 2 receptors, whereas adenylyl cyclase 3 is not receptor-regulated in airway smooth muscle.J Pharmacol Exp Ther. 2012 Aug;342(2):586-95. doi: 10.1124/jpet.112.193425. Epub 2012 May 22. J Pharmacol Exp Ther. 2012. PMID: 22619251 Free PMC article.
-
Human bronchial smooth muscle cells express adenylyl cyclase isoforms 2, 4, and 6 in distinct membrane microdomains.J Pharmacol Exp Ther. 2011 Apr;337(1):209-17. doi: 10.1124/jpet.110.177923. Epub 2011 Jan 12. J Pharmacol Exp Ther. 2011. PMID: 21228062 Free PMC article.
-
PDE8 Is Expressed in Human Airway Smooth Muscle and Selectively Regulates cAMP Signaling by β2-Adrenergic Receptors and Adenylyl Cyclase 6.Am J Respir Cell Mol Biol. 2018 Apr;58(4):530-541. doi: 10.1165/rcmb.2017-0294OC. Am J Respir Cell Mol Biol. 2018. PMID: 29262264 Free PMC article.
-
cAMP Signaling Compartmentation: Adenylyl Cyclases as Anchors of Dynamic Signaling Complexes.Mol Pharmacol. 2018 Apr;93(4):270-276. doi: 10.1124/mol.117.110825. Epub 2017 Dec 7. Mol Pharmacol. 2018. PMID: 29217670 Free PMC article. Review.
-
Physiological roles of mammalian transmembrane adenylyl cyclase isoforms.Physiol Rev. 2022 Apr 1;102(2):815-857. doi: 10.1152/physrev.00013.2021. Epub 2021 Oct 26. Physiol Rev. 2022. PMID: 34698552 Free PMC article. Review.
Cited by
-
Adenylyl cyclase isoforms 5 and 6 in the cardiovascular system: complex regulation and divergent roles.Front Pharmacol. 2024 Apr 3;15:1370506. doi: 10.3389/fphar.2024.1370506. eCollection 2024. Front Pharmacol. 2024. PMID: 38633617 Free PMC article. Review.
-
Discovery of a potent and selective human AC2 inhibitor based on 7-deazapurine analogues of adefovir.Bioorg Med Chem. 2023 Nov 15;95:117508. doi: 10.1016/j.bmc.2023.117508. Epub 2023 Oct 26. Bioorg Med Chem. 2023. PMID: 37931521 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

