Curcumin and its Analogs and Carriers: Potential Therapeutic Strategies for Human Osteosarcoma
- PMID: 36923933
- PMCID: PMC10008701
- DOI: 10.7150/ijbs.80590
Curcumin and its Analogs and Carriers: Potential Therapeutic Strategies for Human Osteosarcoma
Abstract
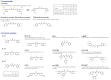
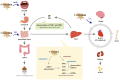
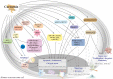
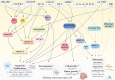
Curcumin is a natural polyphenol phytochemical derived from turmeric with antioxidant, anti-inflammatory, and anticancer properties but is concerned about poor solubility in water, absorption, and metabolic stability. Potent metastatic osteosarcoma is the most common primary bone cancer in children, adolescents, and young adults. It is responsible for low survival rates because of its high rate of metastasis to the lungs. To improve poor bioavailability, numerous curcumin analogs were developed to possess anticancer characteristics through a variety of biological pathways involved in cytotoxicity, proliferation, autophagy, sensitizing chemotherapy, and metastases. This review provides an overview of their various pharmacological functions, molecular mechanisms, and therapeutic potential as a remedy for human osteosarcoma. To enhance therapeutic efficacy, several liposomal nanoparticles, nanocarriers, multifunctional micelles, and three-dimensional printed scaffolds have also been developed for the controlled delivery of curcumin targeting human osteosarcoma cells. Consequently, curcumin and several potential analogs and delivery formulations are optimistic candidates to improve the currently available strategy for human osteosarcoma. However, further insight into the mechanism of action of promising curcumin analogs and the development of carriers in clinical trials of osteosarcoma needs to be investigated to improve their overall potency and clinical utility, in particular the anti-metastatic effect.
Keywords: Bioavailability; carrier; curcumin analogs; human osteosarcoma; therapeutic efficacy.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Curcumin in human osteosarcoma: From analogs to carriers.Drug Discov Today. 2023 Feb;28(2):103437. doi: 10.1016/j.drudis.2022.103437. Epub 2022 Nov 11. Drug Discov Today. 2023. PMID: 36372327 Review.
-
Targeting Signaling Pathway by Curcumin in Osteosarcoma.Curr Mol Pharmacol. 2023;16(1):71-82. doi: 10.2174/1874467215666220408104341. Curr Mol Pharmacol. 2023. PMID: 35400349 Review.
-
Functionalized curcumin analogs as potent modulators of the Wnt/β-catenin signaling pathway.Eur J Med Chem. 2014 Jan;71:67-80. doi: 10.1016/j.ejmech.2013.10.073. Epub 2013 Nov 7. Eur J Med Chem. 2014. PMID: 24275249
-
Selective inhibition of MG-63 osteosarcoma cell proliferation induced by curcumin-loaded self-assembled arginine-rich-RGD nanospheres.Int J Nanomedicine. 2015 May 5;10:3351-65. doi: 10.2147/IJN.S78756. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26005346 Free PMC article.
-
Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering.ACS Appl Mater Interfaces. 2019 May 15;11(19):17184-17192. doi: 10.1021/acsami.9b01218. Epub 2019 May 1. ACS Appl Mater Interfaces. 2019. PMID: 30924639 Free PMC article.
Cited by
-
The current status and future of PD-L1 in liver cancer.Front Immunol. 2023 Dec 12;14:1323581. doi: 10.3389/fimmu.2023.1323581. eCollection 2023. Front Immunol. 2023. PMID: 38155974 Free PMC article. Review.
-
NEK6 Promotes the Progression of Osteosarcoma Through Activating STAT3 Signaling Pathway by Down-Regulation of miR-26a-5p.Int J Gen Med. 2023 Jul 4;16:2831-2848. doi: 10.2147/IJGM.S413461. eCollection 2023. Int J Gen Med. 2023. PMID: 37426517 Free PMC article.
-
Effect of Periodate-Induced Cross-linking on Dual Anticancer Drug Release from Poly(2-isopropyl-2-oxazoline)/Tannic Acid-Based Layer-by-Layer Microparticles.ACS Omega. 2024 Sep 11;9(38):39626-39642. doi: 10.1021/acsomega.4c03977. eCollection 2024 Sep 24. ACS Omega. 2024. PMID: 39346850 Free PMC article.
-
Apoptotic effect and cell arrest of deoxyshikonin in human osteosarcoma cells through the p38 pathway.J Cell Mol Med. 2023 Jun;27(11):1592-1602. doi: 10.1111/jcmm.17764. Epub 2023 May 8. J Cell Mol Med. 2023. PMID: 37155410 Free PMC article.
-
EF-24, a Curcumin Analog, Inhibits Cancer Cell Invasion in Human Nasopharyngeal Carcinoma through Transcriptional Suppression of Matrix Metalloproteinase-9 Gene Expression.Cancers (Basel). 2023 Mar 1;15(5):1552. doi: 10.3390/cancers15051552. Cancers (Basel). 2023. PMID: 36900342 Free PMC article.
References
-
- Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58:2095–9. - PubMed
-
- Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–17. - PubMed
-
- Carter A. Curry compound fights cancer in the clinic. Journal of the National Cancer Institute. 2008;100:616–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

