Attenuation of Alzheimer's brain pathology in 5XFAD mice by PTH1-34, a peptide of parathyroid hormone
- PMID: 36918976
- PMCID: PMC10012528
- DOI: 10.1186/s13195-023-01202-z
Attenuation of Alzheimer's brain pathology in 5XFAD mice by PTH1-34, a peptide of parathyroid hormone
Abstract
Background: Alzheimer's disease (AD) and osteoporosis are two distinct diseases but often occur in the same patient. Their relationship remains poorly understood. Studies using Tg2576 AD animal model demonstrate bone deficits, which precede the brain phenotypes by several months, arguing for the independence of bone deficits on brain degeneration and raising a question if the bone deficits contribute to the AD development. To address this question, we investigated the effects of PTH1-34, a peptide of parathyroid hormone analog and a well-recognized effective anabolic therapy drug for patients with osteoporosis, on 5XFAD animal model.
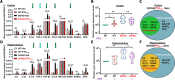
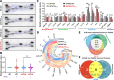
Methods: 5XFAD mice, an early onset β-amyloid (Aβ)-based AD mouse model, were treated with PTH1-34 intermittently [once daily injection of hPTH1-34 (50 μg/Kg), 5 days/week, starting at 2-month old (MO) for 2-3 month]. Wild type mice (C57BL/6) were used as control. The bone phenotypes were examined by microCT and evaluated by measuring serum bone formation and resorption markers. The AD relevant brain pathology (e.g., Aβ and glial activation) and behaviors were assessed by a combination of immunohistochemical staining analysis, western blots, and behavior tests. Additionally, systemic and brain inflammation were evaluated by serum cytokine array, real-time PCR (qPCR), and RNAscope.
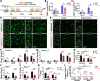
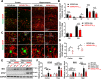
Results: A reduced trabecular, but not cortical, bone mass, accompanied with a decrease in bone formation and an increase in bone resorption, was detected in 5XFAD mice at age of 5/6-month old (MO). Upon PTH1-34 treatments, not only these bone deficits but also Aβ-associated brain pathologies, including Aβ and Aβ deposition levels, dystrophic neurites, glial cell activation, and brain inflammatory cytokines, were all diminished; and the cognitive function was improved. Further studies suggest that PTH1-34 acts on not only osteoblasts in the bone but also astrocytes in the brain, suppressing astrocyte senescence and expression of inflammatory cytokines in 5XFAD mice.
Conclusions: These results suggest that PTH1-34 may act as a senolytic-like drug, reducing systemic and brain inflammation and improving cognitive function, and implicate PTH1-34's therapeutic potential for patients with not only osteoporosis but also AD.
Keywords: Alzheimer’s disease; Astrocytes; Aβ; Neuroinflammation; PTH.
© 2023. The Author(s).
Conflict of interest statement
A patent of this study, entitled “PTH1-34 as a therapeutic drug for Alzheimer’s disease,” has been filed.
Figures



Similar articles
-
Butyrylcholinesterase-knockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer's disease.Brain Res. 2017 Sep 15;1671:102-110. doi: 10.1016/j.brainres.2017.07.009. Epub 2017 Jul 17. Brain Res. 2017. PMID: 28729192
-
MT5-MMP promotes neuroinflammation, neuronal excitability and Aβ production in primary neuron/astrocyte cultures from the 5xFAD mouse model of Alzheimer's disease.J Neuroinflammation. 2022 Mar 11;19(1):65. doi: 10.1186/s12974-022-02407-z. J Neuroinflammation. 2022. PMID: 35277173 Free PMC article.
-
MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer's Disease.Int J Mol Sci. 2018 Jun 18;19(6):1800. doi: 10.3390/ijms19061800. Int J Mol Sci. 2018. PMID: 29912176 Free PMC article.
-
Behaviour Hallmarks in Alzheimer's Disease 5xFAD Mouse Model.Int J Mol Sci. 2024 Jun 20;25(12):6766. doi: 10.3390/ijms25126766. Int J Mol Sci. 2024. PMID: 38928472 Free PMC article. Review.
-
From the Mind to the Spine: The Intersecting World of Alzheimer's and Osteoporosis.Curr Osteoporos Rep. 2024 Feb;22(1):152-164. doi: 10.1007/s11914-023-00848-w. Epub 2024 Feb 9. Curr Osteoporos Rep. 2024. PMID: 38334917 Free PMC article. Review.
Cited by
-
Performance in Behavioral Testing in an Animal Model of Post-Surgical Hypoparathyroidism.J Pers Med. 2024 Feb 17;14(2):215. doi: 10.3390/jpm14020215. J Pers Med. 2024. PMID: 38392648 Free PMC article.
-
Parathyroid Hormone (PTH)-Related Peptides Family: An Intriguing Role in the Central Nervous System.J Pers Med. 2023 Apr 24;13(5):714. doi: 10.3390/jpm13050714. J Pers Med. 2023. PMID: 37240884 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

