Heterologous vaccination with subunit protein vaccine induces a superior neutralizing capacity against BA.4/5-included SARS-CoV-2 variants than homologous vaccination of mRNA vaccine
- PMID: 36911160
- PMCID: PMC10000276
- DOI: 10.1002/mco2.238
Heterologous vaccination with subunit protein vaccine induces a superior neutralizing capacity against BA.4/5-included SARS-CoV-2 variants than homologous vaccination of mRNA vaccine
Abstract
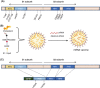
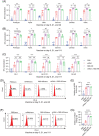
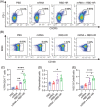
BA.4 and BA.5 (BA.4/5), the subvariants of Omicron, are more transmissible than BA.1 with more robust immune evasion capability because of its unique spike protein mutations. In light of such situation, the vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is in desperate need of the third booster. It has been reported that heterologous boosters might produce more effective immunity against wild-type SARS-CoV-2 and the variants. Additionally, the third heterologous protein subunit booster should be considered potentially. In the present study, we prepared a Delta full-length spike protein sequence-based mRNA vaccine as the "priming" shot and developed a recombinant trimeric receptor-binding domain (RBD) protein vaccine referred to as RBD-HR/trimer as a third heterologous booster. Compared to the homologous mRNA group, the heterologous group (RBD-HR/trimer vaccine primed with two mRNA vaccines) induced higher neutralizing antibody titers against BA.4/5-included SARS-CoV-2 variants. In addition, heterologous vaccination exhibited stronger cellular immune response and long-lasting memory response than the homologous mRNA vaccine. In conclusion, a third heterologous boosting with RBD-HR/trimer following two-dose mRNA priming vaccination should be a superior strategy than a third homologous mRNA vaccine. The RBD-HR/trimer vaccine becomes an appropriate candidate for a booster immune injection.
Keywords: SARS‐CoV‐2; heterologous vaccination; mRNA vaccine; recombinant RBD vaccine.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
This work was supported by the WestVac Biopharma Co. Ltd. Jiong Li, Wei Wang, Guobo Shen, Zhiwei Zhao, Li Yang, Jinliang Yang, Zhenling Wang, Guangwen Lu, and Xiawei Wei are also working at the WestVac Biopharma Co. Ltd. The remaining authors declare no conflict of interests.
Figures


Similar articles
-
Boosting with Multiple Doses of mRNA Vaccine after Priming with Two Doses of Protein Subunit Vaccine MVC-COV1901 Elicited Robust Humoral and Cellular Immune Responses against Emerging SARS-CoV-2 Variants.Microbiol Spectr. 2022 Oct 26;10(5):e0060922. doi: 10.1128/spectrum.00609-22. Epub 2022 Aug 25. Microbiol Spectr. 2022. PMID: 36005765 Free PMC article.
-
Homologous but not heterologous COVID-19 vaccine booster elicits IgG4+ B-cells and enhanced Omicron subvariant binding.NPJ Vaccines. 2024 Jul 17;9(1):129. doi: 10.1038/s41541-024-00919-8. NPJ Vaccines. 2024. PMID: 39013889 Free PMC article.
-
A mosaic-type trimeric RBD-based COVID-19 vaccine candidate induces potent neutralization against Omicron and other SARS-CoV-2 variants.Elife. 2022 Aug 25;11:e78633. doi: 10.7554/eLife.78633. Elife. 2022. PMID: 36004719 Free PMC article.
-
Persistence of immunity against Omicron BA.1 and BA.2 variants following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-dose AZD1222 vaccination.Int J Infect Dis. 2022 Sep;122:793-801. doi: 10.1016/j.ijid.2022.07.038. Epub 2022 Jul 19. Int J Infect Dis. 2022. PMID: 35863731 Free PMC article.
-
Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron.Cytokine Growth Factor Rev. 2023 Apr;70:13-25. doi: 10.1016/j.cytogfr.2023.03.001. Epub 2023 Mar 5. Cytokine Growth Factor Rev. 2023. PMID: 36948931 Free PMC article. Review.
Cited by
-
Intranasal boosting with RBD-HR protein vaccine elicits robust mucosal and systemic immune responses.Genes Dis. 2023 Aug 3;11(4):101066. doi: 10.1016/j.gendis.2023.06.035. eCollection 2024 Jul. Genes Dis. 2023. PMID: 38550714 Free PMC article.
-
Heterologous DNA-prime/protein-boost immunization with a monomeric SARS-CoV-2 spike antigen redundantizes the trimeric receptor-binding domain structure to induce neutralizing antibodies in old mice.Front Immunol. 2023 Sep 11;14:1231274. doi: 10.3389/fimmu.2023.1231274. eCollection 2023. Front Immunol. 2023. PMID: 37753087 Free PMC article.
-
XBB.1.16-RBD-based trimeric protein vaccine can effectively inhibit XBB.1.16-included XBB subvariant infection.MedComm (2020). 2024 Aug 16;5(9):e687. doi: 10.1002/mco2.687. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39156763 Free PMC article.
References
-
- Thakur V, Ratho RK. Omicron (B.1.1.529): a new SARS‐CoV‐2 variant of concern mounting worldwide fear. J Med Virol. 2022;94(5):1821‐1824. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
