Celastrol targeting Nedd4 reduces Nrf2-mediated oxidative stress in astrocytes after ischemic stroke
- PMID: 36908855
- PMCID: PMC9999302
- DOI: 10.1016/j.jpha.2022.12.002
Celastrol targeting Nedd4 reduces Nrf2-mediated oxidative stress in astrocytes after ischemic stroke
Abstract
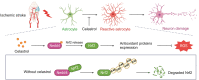
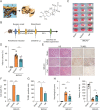
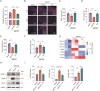
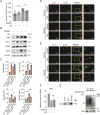
Stroke is the second leading cause of death worldwide, and oxidative stress plays a crucial role. Celastrol exhibits strong antioxidant properties in several diseases; however, whether it can affect oxidation in cerebral ischemic-reperfusion injury (CIRI) remains unclear. This study aimed to determine whether celastrol could reduce oxidative damage during CIRI and to elucidate the underlying mechanisms. Here, we found that celastrol attenuated oxidative injury in CIRI by upregulating nuclear factor E2-related factor 2 (Nrf2). Using alkynyl-tagged celastrol and liquid chromatography-tandem mass spectrometry, we showed that celastrol directly bound to neuronally expressed developmentally downregulated 4 (Nedd4) and then released Nrf2 from Nedd4 in astrocytes. Nedd4 promoted the degradation of Nrf2 through K48-linked ubiquitination and thus contributed to astrocytic reactive oxygen species production in CIRI, which was significantly blocked by celastrol. Furthermore, by inhibiting oxidative stress and astrocyte activation, celastrol effectively rescued neurons from axon damage and apoptosis. Our study uncovered Nedd4 as a direct target of celastrol, and that celastrol exerts an antioxidative effect on astrocytes by inhibiting the interaction between Nedd4 and Nrf2 and reducing Nrf2 degradation in CIRI.
Keywords: Celastrol; Cerebral ischemia; Nedd4; Nrf2; Oxidative stress; Reperfusion injury; Ubiquitylation.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




Similar articles
-
Astragaloside IV mitigates cerebral ischaemia-reperfusion injury via inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis.Eur J Pharmacol. 2023 Apr 5;944:175516. doi: 10.1016/j.ejphar.2023.175516. Epub 2023 Feb 7. Eur J Pharmacol. 2023. PMID: 36758783
-
Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO-1 signaling pathway in mice.Fundam Clin Pharmacol. 2022 Oct;36(5):790-800. doi: 10.1111/fcp.12782. Epub 2022 May 4. Fundam Clin Pharmacol. 2022. PMID: 35470467 Free PMC article.
-
Salidroside inhibited cerebral ischemia/reperfusion-induced oxidative stress and apoptosis via Nrf2/Trx1 signaling pathway.Metab Brain Dis. 2022 Dec;37(8):2965-2978. doi: 10.1007/s11011-022-01061-x. Epub 2022 Aug 17. Metab Brain Dis. 2022. PMID: 35976554
-
Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke.Antioxidants (Basel). 2022 Nov 30;11(12):2377. doi: 10.3390/antiox11122377. Antioxidants (Basel). 2022. PMID: 36552584 Free PMC article. Review.
-
[Research progress on the role of astrocytes in cerebral ischemia/reperfusion injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024 Apr;36(4):441-444. doi: 10.3760/cma.j.cn121430-20230922-00813. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024. PMID: 38813643 Review. Chinese.
Cited by
-
Targeted liposomes for macrophages-mediated pulmonary fibrosis therapy.Drug Deliv Transl Res. 2024 Sep;14(9):2356-2369. doi: 10.1007/s13346-023-01508-3. Epub 2024 Jan 2. Drug Deliv Transl Res. 2024. PMID: 38167826
-
A novel marine-derived anti-acute kidney injury agent targeting peroxiredoxin 1 and its nanodelivery strategy based on ADME optimization.Acta Pharm Sin B. 2024 Jul;14(7):3232-3250. doi: 10.1016/j.apsb.2024.03.005. Epub 2024 Mar 8. Acta Pharm Sin B. 2024. PMID: 39027260 Free PMC article.
-
Complex Inhibitory Activity of Pentacyclic Triterpenoids against Cutaneous Melanoma In Vitro and In Vivo: A Literature Review and Reconstruction of Their Melanoma-Related Protein Interactome.ACS Pharmacol Transl Sci. 2024 Oct 23;7(11):3358-3384. doi: 10.1021/acsptsci.4c00422. eCollection 2024 Nov 8. ACS Pharmacol Transl Sci. 2024. PMID: 39539268 Review.
-
Plant-Based Antioxidants for Prevention and Treatment of Neurodegenerative Diseases: Phytotherapeutic Potential of Laurus nobilis, Aronia melanocarpa, and Celastrol.Antioxidants (Basel). 2023 Mar 18;12(3):746. doi: 10.3390/antiox12030746. Antioxidants (Basel). 2023. PMID: 36978994 Free PMC article. Review.
-
The potential of herbal drugs to treat heart failure: The roles of Sirt1/AMPK.J Pharm Anal. 2024 Feb;14(2):157-176. doi: 10.1016/j.jpha.2023.09.001. Epub 2023 Sep 27. J Pharm Anal. 2024. PMID: 38464786 Free PMC article. Review.
References
-
- Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. - PubMed
LinkOut - more resources
Full Text Sources

