A neural circuit for gastric motility disorders driven by gastric dilation in mice
- PMID: 36908796
- PMCID: PMC9992744
- DOI: 10.3389/fnins.2023.1069198
A neural circuit for gastric motility disorders driven by gastric dilation in mice
Abstract
Introduction: Symptoms of gastric motility disorders are common clinical manifestations of functional gastrointestinal disorders (FGIDs), and are triggered and exacerbated by stress, but the neural pathways underpinning them remain unclear.
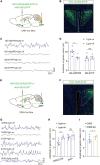
Methods: We set-up a mouse model by gastric dilation (GD) in which the gastric dynamics were assessed by installing strain gauges on the surface of the stomach. The neural pathway associated with gastric motility disorders was investigated by behavioral tests, electrophysiology, neural circuit tracing, and optogenetics and chemogenetics involving projections of the corticotropin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus (PVN) to acetylcholine (ChAT) neurons in the dorsal motor nucleus of the vagus (DMV).
Results: We found that GD induced gastric motility disorders were accompanied by activation of PVN CRH neurons, which could be alleviated by strategies that inhibits the activity of PVN CRH neurons. In addition, we identified a neural pathway in which PVN CRH neurons project into DMV ChAT neurons, modulated activity of the PVN CRH →DMV ChAT pathway to alleviate gastric motility disorders induced by GD.
Discussion: These findings indicate that the PVN CRH →DMV ChAT pathway may mediate at least some aspects of GD related gastric motility, and provide new insights into the mechanisms by which somatic stimulation modulates the physiological functions of internal organs and systems.
Keywords: corticotropin-releasing hormone; dorsal motor nucleus of the vagus; gastric motility disorders; neural circuit; paraventricular nucleus of the hypothalamus.
Copyright © 2023 Wang, Chen, Wang, Cai, Wang, Wang, Peng, Zhang, Huang and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Perinatal high-fat diet exposure alters oxytocin and corticotropin releasing factor inputs onto vagal neurocircuits controlling gastric motility.J Physiol. 2023 Jul;601(14):2853-2875. doi: 10.1113/JP284726. Epub 2023 May 17. J Physiol. 2023. PMID: 37154244 Free PMC article.
-
Corticotropin-releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increase blood pressure.J Neurophysiol. 2019 Feb 1;121(2):602-608. doi: 10.1152/jn.00623.2018. Epub 2018 Dec 19. J Neurophysiol. 2019. PMID: 30565964 Free PMC article.
-
Reward Inhibits Paraventricular CRH Neurons to Relieve Stress.Curr Biol. 2019 Apr 1;29(7):1243-1251.e4. doi: 10.1016/j.cub.2019.02.048. Epub 2019 Mar 7. Curr Biol. 2019. PMID: 30853436
-
Heterosynaptic modulation in the paraventricular nucleus of the hypothalamus.Neuropharmacology. 2019 Aug;154:87-95. doi: 10.1016/j.neuropharm.2018.11.004. Epub 2018 Nov 5. Neuropharmacology. 2019. PMID: 30408488 Review.
-
Computational models of autonomic regulation in gastric motility: Progress, challenges, and future directions.Front Neurosci. 2023 Mar 15;17:1146097. doi: 10.3389/fnins.2023.1146097. eCollection 2023. Front Neurosci. 2023. PMID: 37008202 Free PMC article. Review.
Cited by
-
Postoperative delayed gastric emptying: may gut microbiota play a role?Front Cell Infect Microbiol. 2024 Aug 13;14:1449530. doi: 10.3389/fcimb.2024.1449530. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39193506 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

