TCF7L1 Accelerates Smooth Muscle Cell Phenotypic Switching and Aggravates Abdominal Aortic Aneurysms
- PMID: 36908661
- PMCID: PMC9998605
- DOI: 10.1016/j.jacbts.2022.07.012
TCF7L1 Accelerates Smooth Muscle Cell Phenotypic Switching and Aggravates Abdominal Aortic Aneurysms
Abstract
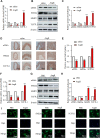
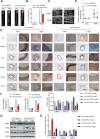
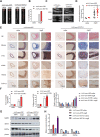
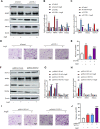
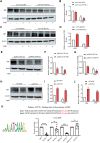
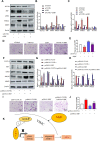
Phenotypic switching of vascular smooth muscle cells is a central process in abdominal aortic aneurysm (AAA) pathology. We found that knockdown TCF7L1 (transcription factor 7-like 1), a member of the TCF/LEF (T cell factor/lymphoid enhancer factor) family of transcription factors, inhibits vascular smooth muscle cell differentiation. This study hints at potential interventions to maintain a normal, differentiated smooth muscle cell state, thereby eliminating the pathogenesis of AAA. In addition, our study provides insights into the potential use of TCF7L1 as a biomarker for AAA.
Keywords: AAA, abdominal aortic aneurysm; AAV, adeno-associated virus; Ang II, angiotensin II; CVF, collagen volume fraction; MMP, matrix metalloproteinase; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; SM22α, smooth muscle protein 22-α; SMA, smooth muscle actin; SRF, serum response factor; TCF7L1; TCF7L1, transcription factor 7-like 1; VSMC, vascular smooth muscle cell; abdominal aortic aneurysms; cDNA, complementary DNA; mRNA, messenger RNA; phenotypic switching; siRNA, small interfering RNA; smooth muscle cell.
© 2023 The Authors.
Conflict of interest statement
This work was supported by the National Natural Science Foundation of China (81870553, 82070308, 82070300, and 82070875), the Excellent Youth Fund of Liaoning Natural Science Foundation (2021-YQ-03), and the Shenyang Science and Technology Project (20-205-4-001). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Similar articles
-
SM22α (Smooth Muscle 22α) Prevents Aortic Aneurysm Formation by Inhibiting Smooth Muscle Cell Phenotypic Switching Through Suppressing Reactive Oxygen Species/NF-κB (Nuclear Factor-κB).Arterioscler Thromb Vasc Biol. 2019 Jan;39(1):e10-e25. doi: 10.1161/ATVBAHA.118.311917. Arterioscler Thromb Vasc Biol. 2019. PMID: 30580562
-
TRPV5 attenuates abdominal aortic aneurysm in mice by regulating KLF4-dependent phenotype switch of aortic vascular smooth muscle cells.Arch Biochem Biophys. 2021 Feb 15;698:108724. doi: 10.1016/j.abb.2020.108724. Epub 2020 Dec 11. Arch Biochem Biophys. 2021. PMID: 33309615
-
Loss of FoxO3a prevents aortic aneurysm formation through maintenance of VSMC homeostasis.Cell Death Dis. 2021 Apr 7;12(4):378. doi: 10.1038/s41419-021-03659-y. Cell Death Dis. 2021. PMID: 33828087 Free PMC article.
-
Abdominal Aortic Aneurysm Formation with a Focus on Vascular Smooth Muscle Cells.Life (Basel). 2022 Jan 27;12(2):191. doi: 10.3390/life12020191. Life (Basel). 2022. PMID: 35207478 Free PMC article. Review.
-
Single-cell RNA sequencing provides novel insights to pathologic pathways in abdominal aortic aneurysm.Front Cardiovasc Med. 2023 May 23;10:1172080. doi: 10.3389/fcvm.2023.1172080. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37288252 Free PMC article. Review.
Cited by
-
Activation of nuclear receptor pregnane-X-receptor protects against abdominal aortic aneurysm by inhibiting oxidative stress.Redox Biol. 2024 Nov;77:103397. doi: 10.1016/j.redox.2024.103397. Epub 2024 Oct 17. Redox Biol. 2024. PMID: 39427444 Free PMC article.
-
TCF7L1 Exacerbates Abdominal Aortic Aneurysm Prevalence and Severity.JACC Basic Transl Sci. 2023 Feb 27;8(2):171-173. doi: 10.1016/j.jacbts.2022.09.006. eCollection 2023 Feb. JACC Basic Transl Sci. 2023. PMID: 36908666 Free PMC article.
-
Inhibition of smooth muscle cell death by Angiotensin 1-7 protects against abdominal aortic aneurysm.Biosci Rep. 2023 Nov 30;43(11):BSR20230718. doi: 10.1042/BSR20230718. Biosci Rep. 2023. PMID: 37947205 Free PMC article.
References
-
- Benjamin E.J., Virani S.S., Callawawy C.W., et al. Heart Disease and Stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. - PubMed
-
- Sakalihasan N., Limet R., Defawe O.D. Abdominal aortic aneurysm. Lancet. 2005;365:1577–1589. - PubMed
-
- Zhong L., He X., Si X., et al. SM22alpha (smooth muscle 22alpha) prevents aortic aneurysm formation by inhibiting smooth muscle cell phenotypic switching through suppressing reactive oxygen species/NF-kappaB (nuclear factor-kappaB) Arterioscler Thromb Vasc Biol. 2019;39:e10–e25. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

