Relapsed/Refractory Chronic Lymphocytic Leukemia Patients Treated with Fixed Duration Venetoclax-Rituximab: Assessment of Response with Ultrasound, and Relationship with Minimal Residual Disease
- PMID: 36902559
- PMCID: PMC10003523
- DOI: 10.3390/jcm12051772
Relapsed/Refractory Chronic Lymphocytic Leukemia Patients Treated with Fixed Duration Venetoclax-Rituximab: Assessment of Response with Ultrasound, and Relationship with Minimal Residual Disease
Abstract
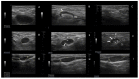
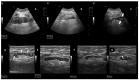
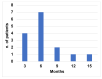
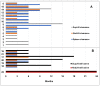
A fixed duration of venetoclax-rituximab (VenR) resulted in a significant benefit of both PFS and in the attainment of an undetectable minimal residual disease (uMRD) compared with bendamustine-rituximab in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) patients. The 2018 International Workshop on CLL guidelines, outside the context of clinical trials, suggested ultrasonography (US) as a possible imaging technique to evaluate visceral involvement, and palpation to evaluate superficial lymph nodes (SupLNs). In this real-life study we prospectively enrolled N = 22 patients. Patients were assessed by US, to determine nodal and splenic response in R/R CLL patients treated with a fixed duration VenR. We found an overall response rate, complete remission, partial remission, and stable disease, of 95.4%, 68%, 27.3%, and 4.5%, respectively. Responses were also correlated with risk categories. The time to response, and the time to clearance of the disease in the spleen, in abdominal LN (AbdLNs), and in SupLNs were discussed. Responses were independent from LN size. The correlation between response rate with MRD were also investigated. US allowed to detect a substantial CR rate correlated with uMRD.
Keywords: chronic lymphocytic leukemia; ultrasound sonography; venetoclax-rituximab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab.Blood. 2022 Aug 25;140(8):839-850. doi: 10.1182/blood.2021015014. Blood. 2022. PMID: 35605176 Free PMC article. Clinical Trial.
-
Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations From the MURANO Phase III Study.J Clin Oncol. 2020 Dec 1;38(34):4042-4054. doi: 10.1200/JCO.20.00948. Epub 2020 Sep 28. J Clin Oncol. 2020. PMID: 32986498 Free PMC article. Clinical Trial.
-
Fixed Duration of Venetoclax-Rituximab in Relapsed/Refractory Chronic Lymphocytic Leukemia Eradicates Minimal Residual Disease and Prolongs Survival: Post-Treatment Follow-Up of the MURANO Phase III Study.J Clin Oncol. 2019 Feb 1;37(4):269-277. doi: 10.1200/JCO.18.01580. Epub 2018 Dec 3. J Clin Oncol. 2019. PMID: 30523712 Clinical Trial.
-
Minimal Residual Disease in Chronic Lymphocytic Leukemia: A New Goal?Front Oncol. 2019 Aug 29;9:689. doi: 10.3389/fonc.2019.00689. eCollection 2019. Front Oncol. 2019. PMID: 31555576 Free PMC article. Review.
-
BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia.Curr Treat Options Oncol. 2021 Jun 10;22(8):66. doi: 10.1007/s11864-021-00862-z. Curr Treat Options Oncol. 2021. PMID: 34110507 Review.
Cited by
-
Real-life diagnostic and therapeutic approach to CLL: a 2022 update from an expert panel in Tuscany.Clin Exp Med. 2023 Dec;23(8):4251-4264. doi: 10.1007/s10238-023-01244-5. Epub 2023 Nov 18. Clin Exp Med. 2023. PMID: 37979127 Review.
References
-
- Eichhorst B., Robak T., Montserrat E., Ghia P., Niemann C.U., Kater A.P., Gregor M., Cymbalista F., Buske C., Hillmen P., et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021;32:23–33. doi: 10.1016/j.annonc.2020.09.019. - DOI - PubMed

