Update on the Pathogenesis of the Hirschsprung-Associated Enterocolitis
- PMID: 36902033
- PMCID: PMC10003052
- DOI: 10.3390/ijms24054602
Update on the Pathogenesis of the Hirschsprung-Associated Enterocolitis
Abstract
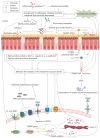
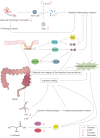
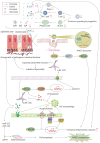
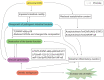
Despite the significant progress that has been made in terms of understanding the pathophysiology and risk factors of Hirschsprung-associated enterocolitis (HAEC), the morbidity rate has remained unsatisfactorily stable, and clinical management of the condition continues to be challenging. Therefore, in the present literature review, we summarized the up-to-date advances that have been made regarding basic research on the pathogenesis of HAEC. Original articles published between August 2013 and October 2022 were searched in a number of databases, including PubMed, Web of Science, and Scopus. The keywords "Hirschsprung enterocolitis", "Hirschsprung's enterocolitis", "Hirschsprung's-associated enterocolitis", and "Hirschsprung-associated enterocolitis" were selected and reviewed. A total of 50 eligible articles were obtained. The latest findings of these research articles were grouped into gene, microbiome, barrier function, enteric nervous system, and immune state categories. The present review concludes that HAEC is shown to be a multifactorial clinical syndrome. Only deep insights into this syndrome, with an accrual of knowledge in terms of understanding its pathogenesis, will elicit the necessary changes that are required for managing this disease.
Keywords: Hirschsprung disease (HSCR); Hirschsprung-associated enterocolitis (HAEC); enterocolitis; pathogenesis; review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Novel Insights into the Pathogenesis of Hirschsprung's-associated Enterocolitis.Chin Med J (Engl). 2016 Jun 20;129(12):1491-7. doi: 10.4103/0366-6999.183433. Chin Med J (Engl). 2016. PMID: 27270548 Free PMC article. Review.
-
Probiotics for the prevention of Hirschsprung-associated enterocolitis: a systematic review and meta-analysis.Pediatr Surg Int. 2018 Feb;34(2):189-193. doi: 10.1007/s00383-017-4188-y. Epub 2017 Oct 5. Pediatr Surg Int. 2018. PMID: 28983778 Review.
-
The pathogenesis of Hirschsprung's disease-associated enterocolitis.Semin Pediatr Surg. 2012 Nov;21(4):319-27. doi: 10.1053/j.sempedsurg.2012.07.006. Semin Pediatr Surg. 2012. PMID: 22985837 Review.
-
Inflammatory bowel disease in patients with Hirschsprung's disease: a systematic review and meta-analysis.Pediatr Surg Int. 2018 Feb;34(2):149-154. doi: 10.1007/s00383-017-4182-4. Epub 2017 Oct 5. Pediatr Surg Int. 2018. PMID: 28983688 Review.
-
Altered tryptophan hydroxylase 2 expression in enteric serotonergic nerves in Hirschsprung's-associated enterocolitis.World J Gastroenterol. 2016 May 21;22(19):4662-72. doi: 10.3748/wjg.v22.i19.4662. World J Gastroenterol. 2016. PMID: 27217698 Free PMC article.
Cited by
-
A unicentric cross-sectional observational study on chronic intestinal inflammation in total colonic aganglionosis: beware of an underestimated condition.Orphanet J Rare Dis. 2023 Oct 27;18(1):339. doi: 10.1186/s13023-023-02958-1. Orphanet J Rare Dis. 2023. PMID: 37891621 Free PMC article.
-
The roles of non-coding RNAs in Hirschsprung's disease.Noncoding RNA Res. 2024 Feb 28;9(3):704-714. doi: 10.1016/j.ncrna.2024.02.015. eCollection 2024 Sep. Noncoding RNA Res. 2024. PMID: 38577013 Free PMC article. Review.
-
Angiopoietin-1 attenuates lipopolysaccharide-induced endotoxemia in a Hirschsprung's disease murine model by improving intestinal vascular integrity: implications for treating postoperative Hirschsprung-associated enterocolitis.Pediatr Surg Int. 2024 Oct 28;40(1):277. doi: 10.1007/s00383-024-05867-x. Pediatr Surg Int. 2024. PMID: 39466437
-
Clinical characteristics and influence of postoperative Hirschsprung-associated enterocolitis: retrospective study at a tertiary children's hospital.Pediatr Surg Int. 2024 Apr 13;40(1):106. doi: 10.1007/s00383-024-05688-y. Pediatr Surg Int. 2024. PMID: 38613719
-
Efficient enzyme-free method to assess the development and maturation of the innate and adaptive immune systems in the mouse colon.Sci Rep. 2024 May 14;14(1):11063. doi: 10.1038/s41598-024-61834-5. Sci Rep. 2024. PMID: 38744932 Free PMC article.
References
-
- Langer J.C., Rollins M.D., Levitt M., Gosain A., de la Torre L., Kapur R.P., Cowles R.A., Horton J., Rothstein D.H., Goldstein A.M., et al. Guidelines for the management of postoperative obstructive symptoms in children with Hirschsprung disease. Pediatr. Surg. Int. 2017;33:523–526. doi: 10.1007/s00383-017-4066-7. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

