Context-Dependent Role of Glucocorticoid Receptor Alpha and Beta in Breast Cancer Cell Behaviour
- PMID: 36899920
- PMCID: PMC10000936
- DOI: 10.3390/cells12050784
Context-Dependent Role of Glucocorticoid Receptor Alpha and Beta in Breast Cancer Cell Behaviour
Abstract
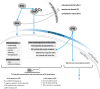
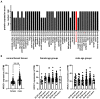
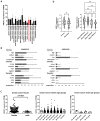
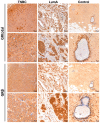
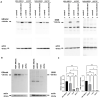
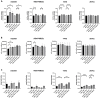
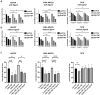
Background. The dual role of GCs has been observed in breast cancer; however, due to many concomitant factors, GR action in cancer biology is still ambiguous. In this study, we aimed to unravel the context-dependent action of GR in breast cancer. Methods. GR expression was characterized in multiple cohorts: (1) 24,256 breast cancer specimens on the RNA level, 220 samples on the protein level and correlated with clinicopathological data; (2) oestrogen receptor (ER)-positive and -negative cell lines were used to test for the presence of ER and ligand, and the effect of the GRβ isoform following GRα and GRβ overexpression on GR action, by in vitro functional assays. Results. We found that GR expression was higher in ER- breast cancer cells compared to ER+ ones, and GR-transactivated genes were implicated mainly in cell migration. Immunohistochemistry showed mostly cytoplasmic but heterogenous staining irrespective of ER status. GRα increased cell proliferation, viability, and the migration of ER- cells. GRβ had a similar effect on breast cancer cell viability, proliferation, and migration. However, the GRβ isoform had the opposite effect depending on the presence of ER: an increased dead cell ratio was found in ER+ breast cancer cells compared to ER- ones. Interestingly, GRα and GRβ action did not depend on the presence of the ligand, suggesting the role of the "intrinsic", ligand-independent action of GR in breast cancer. Conclusions. Staining differences using different GR antibodies may be the reason behind controversial findings in the literature regarding the expression of GR protein and clinicopathological data. Therefore, caution in the interpretation of immunohistochemistry should be applied. By dissecting the effects of GRα and GRβ, we found that the presence of the GR in the context of ER had a different effect on cancer cell behaviour, but independently of ligand availability. Additionally, GR-transactivated genes are mostly involved in cell migration, which raises GR's importance in disease progression.
Keywords: breast cancer; breast cancer progression; glucocorticoid receptor; glucocorticoid receptor alpha; glucocorticoid receptor beta; metastasis; migration; proliferation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Glucocorticoid Receptor β (GRβ): Beyond Its Dominant-Negative Function.Int J Mol Sci. 2021 Mar 31;22(7):3649. doi: 10.3390/ijms22073649. Int J Mol Sci. 2021. PMID: 33807481 Free PMC article. Review.
-
Relationship between expression of glucocorticoid receptor isoforms and glucocorticoid resistance in immune thrombocytopenia.Hematology. 2016 Aug;21(7):440-6. doi: 10.1080/10245332.2015.1102371. Epub 2016 Mar 21. Hematology. 2016. PMID: 27077774
-
Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish.Endocrinology. 2008 Apr;149(4):1591-9. doi: 10.1210/en.2007-1364. Epub 2007 Dec 20. Endocrinology. 2008. PMID: 18096659
-
Interindividual glucocorticoid sensitivity in young healthy subjects: the role of glucocorticoid receptor alpha and beta isoforms ratio.Horm Metab Res. 2007 Jun;39(6):425-9. doi: 10.1055/s-2007-980191. Horm Metab Res. 2007. PMID: 17578759
-
Is there a role for glucocorticoid receptor beta in asthma?Respir Res. 2001;2(1):1-4. doi: 10.1186/rr31. Epub 2000 Dec 18. Respir Res. 2001. PMID: 11686858 Free PMC article. Review.
References
-
- Lamb C.A., Vanzulli S.I., Lanari C. Hormone Receptors in Breast Cancer: More than Estrogen Receptors. Medicina. 2019;79:540–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

