Emerging roles of endoplasmic reticulum stress in the cellular plasticity of cancer cells
- PMID: 36890838
- PMCID: PMC9986440
- DOI: 10.3389/fonc.2023.1110881
Emerging roles of endoplasmic reticulum stress in the cellular plasticity of cancer cells
Abstract
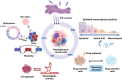
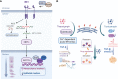
Cellular plasticity is a well-known dynamic feature of tumor cells that endows tumors with heterogeneity and therapeutic resistance and alters their invasion-metastasis progression, stemness, and drug sensitivity, thereby posing a major challenge to cancer therapy. It is becoming increasingly clear that endoplasmic reticulum (ER) stress is a hallmark of cancer. The dysregulated expression of ER stress sensors and the activation of downstream signaling pathways play a role in the regulation of tumor progression and cellular response to various challenges. Moreover, mounting evidence implicates ER stress in the regulation of cancer cell plasticity, including epithelial-mesenchymal plasticity, drug resistance phenotype, cancer stem cell phenotype, and vasculogenic mimicry phenotype plasticity. ER stress influences several malignant characteristics of tumor cells, including epithelial-to-mesenchymal transition (EMT), stem cell maintenance, angiogenic function, and tumor cell sensitivity to targeted therapy. The emerging links between ER stress and cancer cell plasticity that are implicated in tumor progression and chemoresistance are discussed in this review, which may aid in formulating strategies to target ER stress and cancer cell plasticity in anticancer treatments.
Keywords: ER stress; cancer stem cell; cellular plasticity; epithelial-mesenchymal plasticity; resistance; vasculogenic mimicry.
Copyright © 2023 Wang and Mi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Communication Between Epithelial-Mesenchymal Plasticity and Cancer Stem Cells: New Insights Into Cancer Progression.Front Oncol. 2021 Apr 21;11:617597. doi: 10.3389/fonc.2021.617597. eCollection 2021. Front Oncol. 2021. PMID: 33968721 Free PMC article. Review.
-
Emerging roles of epithelial-mesenchymal plasticity in invasion-metastasis cascade and therapy resistance.Cancer Metastasis Rev. 2022 Mar;41(1):131-145. doi: 10.1007/s10555-021-10003-5. Epub 2022 Jan 3. Cancer Metastasis Rev. 2022. PMID: 34978017 Review.
-
Epithelial-to-mesenchymal transition activates PERK-eIF2α and sensitizes cells to endoplasmic reticulum stress.Cancer Discov. 2014 Jun;4(6):702-15. doi: 10.1158/2159-8290.CD-13-0945. Epub 2014 Apr 4. Cancer Discov. 2014. PMID: 24705811
-
Inhibitory effect of melatonin on hypoxia-induced vasculogenic mimicry via suppressing epithelial-mesenchymal transition (EMT) in breast cancer stem cells.Eur J Pharmacol. 2020 Aug 15;881:173282. doi: 10.1016/j.ejphar.2020.173282. Epub 2020 Jun 21. Eur J Pharmacol. 2020. PMID: 32580038
-
Harnessing Carcinoma Cell Plasticity Mediated by TGF-β Signaling.Cancers (Basel). 2021 Jul 7;13(14):3397. doi: 10.3390/cancers13143397. Cancers (Basel). 2021. PMID: 34298613 Free PMC article. Review.
Cited by
-
Unveiling the mechanisms and challenges of cancer drug resistance.Cell Commun Signal. 2024 Feb 12;22(1):109. doi: 10.1186/s12964-023-01302-1. Cell Commun Signal. 2024. PMID: 38347575 Free PMC article. Review.
-
ER Stress-Activated HSF1 Governs Cancer Cell Resistance to USP7 Inhibitor-Based Chemotherapy through the PERK Pathway.Int J Mol Sci. 2024 Feb 27;25(5):2768. doi: 10.3390/ijms25052768. Int J Mol Sci. 2024. PMID: 38474017 Free PMC article.
-
Chronic arsenic exposure induces malignant transformation of human HaCaT cells through both deterministic and stochastic changes in transcriptome expression.Toxicol Appl Pharmacol. 2024 Mar;484:116865. doi: 10.1016/j.taap.2024.116865. Epub 2024 Feb 17. Toxicol Appl Pharmacol. 2024. PMID: 38373578
-
ORP5 promotes migration and invasion of cervical cancer cells by inhibiting endoplasmic reticulum stress.Cell Stress Chaperones. 2023 Jul;28(4):395-407. doi: 10.1007/s12192-023-01357-6. Epub 2023 Jun 14. Cell Stress Chaperones. 2023. PMID: 37314629 Free PMC article.
-
A novel gene signature based on endoplasmic reticulum stress for predicting prognosis in hepatocellular carcinoma.Transl Cancer Res. 2024 Sep 30;13(9):4574-4592. doi: 10.21037/tcr-24-191. Epub 2024 Sep 18. Transl Cancer Res. 2024. PMID: 39430815 Free PMC article.
References
-
- Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov (2019) 9:837–51. doi: 10.1158/2159-8290.CD-19-0015 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

