Can cartilage intermediate layer protein 1 (CILP1) use as a novel biomarker for canine myxomatous mitral valve degeneration levels or not?
- PMID: 36882760
- PMCID: PMC9990206
- DOI: 10.1186/s12917-023-03583-7
Can cartilage intermediate layer protein 1 (CILP1) use as a novel biomarker for canine myxomatous mitral valve degeneration levels or not?
Abstract
Background: Myxomatous mitral valve degeneration (MMVD) is the most common degenerative heart disease in dogs and is associated with irreversible changes in the valve tissue. Although traditional cardiac biomarkers are efficient for diagnosing MMVD, there are limitations, therefore, it is important to find novel biomarkers. Cartilage intermediate layer protein 1 (CILP1), an extracellular matrix-derived protein, acts as a transforming growth factor-β antagonist and is involved in myocardial fibrosis. This study aimed to evaluate serum CILP1 levels in canines with MMVD. Dogs with MMVD were staged according to the American College of Veterinary Internal Medicine consensus guidelines. Data analysis was performed using the Mann-Whitney U test, Spearman's correlation, and receiver operating characteristic (ROC) curves.
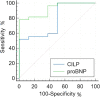
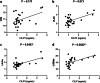
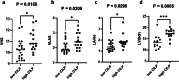
Results: CILP1 levels were elevated in dogs with MMVD (n = 27) compared to healthy controls (n = 8). Furthermore, results showed that CILP1 levels were significantly higher in stage C group dogs compared to healthy controls. The ROC curve of CILP1 and NT-proBNP were good predictors of MMVD, although no similarity was observed between the two. Left ventricular end-diastolic diameter normalized to the body weight (LVIDdn) and left atrial to aorta dimension (LA/Ao) showed a strong association with CILP1 levels; however, no correlation was observed between CILP1 levels and vertebral heart size (VHS) and vertebral left atrial score (VLAS). The optimal cut-off value was selected from the ROC curve and dogs were classified according to the cut-off value (1.068 ng/mL, sensitivity 51.9%, specificity 100%). Results showed a significant association of CILP1 with cardiac remodeling indicators, such as VHS, VLAS, LA/Ao, and LVIDdn.
Conclusions: CILP1 can be an indicator of cardiac remodeling in canines with MMVD and therefore, can be used as an MMVD biomarker.
Keywords: Biomarker; Cardiac; Cartilage intermediate layer protein 1; Dogs; Myxomatous mitral valve degeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Two radiographic methods for assessing left atrial enlargement and cardiac remodeling in dogs with myxomatous mitral valve disease.J Vet Cardiol. 2021 Apr;34:55-63. doi: 10.1016/j.jvc.2021.01.002. Epub 2021 Jan 15. J Vet Cardiol. 2021. PMID: 33581663
-
Diagnostic value of atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and their correlation with lipoproteins in dogs with myxomatous mitral valve disease.BMC Vet Res. 2022 Dec 23;18(1):448. doi: 10.1186/s12917-022-03548-2. BMC Vet Res. 2022. PMID: 36564735 Free PMC article.
-
Radiographic quantification of left atrial size in dogs with myxomatous mitral valve disease.J Vet Intern Med. 2021 Mar;35(2):747-754. doi: 10.1111/jvim.16073. Epub 2021 Feb 26. J Vet Intern Med. 2021. PMID: 33634912 Free PMC article.
-
Advances in the understanding of the pathogenesis, progression and diagnosis of myxomatous mitral valve disease in dogs.J S Afr Vet Assoc. 2014 Aug 13;85(1):1101. doi: 10.4102/jsava.v85i1.1101. J S Afr Vet Assoc. 2014. PMID: 25685978 Review.
-
Comparative pathology of human and canine myxomatous mitral valve degeneration: 5HT and TGF-β mechanisms.Cardiovasc Pathol. 2020 May-Jun;46:107196. doi: 10.1016/j.carpath.2019.107196. Epub 2020 Jan 7. Cardiovasc Pathol. 2020. PMID: 32006823 Free PMC article. Review.
References
-
- Thrusfield MV, Aitken CGG, Darke PGG. Observations on breed and sex in relation to canine heart valve incompetence. J Small Anim Pract. 1985. 10.1111/j.1748-5827.1985.tb02199.x.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

