Septins as membrane influencers: direct play or in association with other cytoskeleton partners
- PMID: 36875762
- PMCID: PMC9982393
- DOI: 10.3389/fcell.2023.1112319
Septins as membrane influencers: direct play or in association with other cytoskeleton partners
Abstract
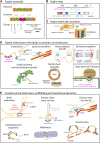
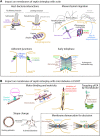
The cytoskeleton comprises three polymerizing structures that have been studied for a long time, actin microfilaments, microtubules and intermediate filaments, plus more recently investigated dynamic assemblies like septins or the endocytic-sorting complex required for transport (ESCRT) complex. These filament-forming proteins control several cell functions through crosstalks with each other and with membranes. In this review, we report recent works that address how septins bind to membranes, and influence their shaping, organization, properties and functions, either by binding to them directly or indirectly through other cytoskeleton elements.
Keywords: ESCRT; actin; cytoskeleton; intermediate filament; membrane; microtubule; septin.
Copyright © 2023 Benoit, Poüs and Baillet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cytoskeleton and Associated Proteins: Pleiotropic JNK Substrates and Regulators.Int J Mol Sci. 2021 Aug 4;22(16):8375. doi: 10.3390/ijms22168375. Int J Mol Sci. 2021. PMID: 34445080 Free PMC article. Review.
-
Actin, microtubule, septin and ESCRT filament remodeling during late steps of cytokinesis.Curr Opin Cell Biol. 2018 Feb;50:27-34. doi: 10.1016/j.ceb.2018.01.007. Epub 2018 Feb 10. Curr Opin Cell Biol. 2018. PMID: 29438904 Review.
-
Septins mediate a microtubule-actin crosstalk that enables actin growth on microtubules.Proc Natl Acad Sci U S A. 2022 Dec 13;119(50):e2202803119. doi: 10.1073/pnas.2202803119. Epub 2022 Dec 7. Proc Natl Acad Sci U S A. 2022. PMID: 36475946 Free PMC article.
-
Cellular functions of actin- and microtubule-associated septins.Curr Biol. 2021 May 24;31(10):R651-R666. doi: 10.1016/j.cub.2021.03.064. Curr Biol. 2021. PMID: 34033796 Free PMC article. Review.
-
Submembranous septins as relatively stable components of actin-based membrane skeleton.Cytoskeleton (Hoboken). 2011 Sep;68(9):512-25. doi: 10.1002/cm.20528. Epub 2011 Aug 25. Cytoskeleton (Hoboken). 2011. PMID: 21800439
Cited by
-
The septin cytoskeleton is required for plasma membrane repair.EMBO Rep. 2024 Sep;25(9):3870-3895. doi: 10.1038/s44319-024-00195-6. Epub 2024 Jul 5. EMBO Rep. 2024. PMID: 38969946 Free PMC article.
-
Septins as key players in spermatogenesis, fertilisation and pre-implantation embryogenic cytoplasmic dynamics.Cell Commun Signal. 2024 Oct 28;22(1):523. doi: 10.1186/s12964-024-01889-z. Cell Commun Signal. 2024. PMID: 39468561 Free PMC article. Review.
-
Septin-coated microtubules promote maturation of multivesicular bodies by inhibiting their motility.J Cell Biol. 2024 Aug 5;223(8):e202308049. doi: 10.1083/jcb.202308049. Epub 2024 Apr 26. J Cell Biol. 2024. PMID: 38668767 Free PMC article.
-
SEPT9_i1 and Septin Dynamics in Oncogenesis and Cancer Treatment.Biomolecules. 2024 Sep 22;14(9):1194. doi: 10.3390/biom14091194. Biomolecules. 2024. PMID: 39334960 Free PMC article. Review.
-
Animal septins contain functional transmembrane domains.bioRxiv [Preprint]. 2024 Aug 29:2023.11.20.567915. doi: 10.1101/2023.11.20.567915. bioRxiv. 2024. PMID: 38045322 Free PMC article. Preprint.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

