Targeted PERK inhibition with biomimetic nanoclusters confers preventative and interventional benefits to elastase-induced abdominal aortic aneurysms
- PMID: 36875050
- PMCID: PMC9975632
- DOI: 10.1016/j.bioactmat.2023.02.009
Targeted PERK inhibition with biomimetic nanoclusters confers preventative and interventional benefits to elastase-induced abdominal aortic aneurysms
Abstract
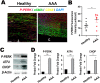
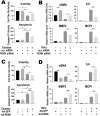
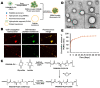
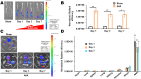
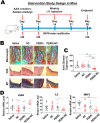
Abdominal aortic aneurysm (AAA) is a progressive aortic dilatation, causing ∼80% mortality upon rupture. Currently, there is no approved drug therapy for AAA. Surgical repairs are invasive and risky and thus not recommended to patients with small AAAs which, however, account for ∼90% of the newly diagnosed cases. It is therefore a compelling unmet clinical need to discover effective non-invasive strategies to prevent or slow down AAA progression. We contend that the first AAA drug therapy will only arise through discoveries of both effective drug targets and innovative delivery methods. There is substantial evidence that degenerative smooth muscle cells (SMCs) orchestrate AAA pathogenesis and progression. In this study, we made an exciting finding that PERK, the endoplasmic reticulum (ER) stress Protein Kinase R-like ER Kinase, is a potent driver of SMC degeneration and hence a potential therapeutic target. Indeed, local knockdown of PERK in elastase-challenged aorta significantly attenuated AAA lesions in vivo. In parallel, we also conceived a biomimetic nanocluster (NC) design uniquely tailored to AAA-targeting drug delivery. This NC demonstrated excellent AAA homing via a platelet-derived biomembrane coating; and when loaded with a selective PERK inhibitor (PERKi, GSK2656157), the NC therapy conferred remarkable benefits in both preventing aneurysm development and halting the progression of pre-existing aneurysmal lesions in two distinct rodent models of AAA. In summary, our current study not only establishes a new intervention target for mitigating SMC degeneration and aneurysmal pathogenesis, but also provides a powerful tool to facilitate the development of effective drug therapy of AAA.
Keywords: Abdominal aortic aneurysm; Biomimetic nanomedicine; ER stress; PERK; Targeted delivery.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Endovascular repair of abdominal aortic aneurysm: an evidence-based analysis.Ont Health Technol Assess Ser. 2002;2(1):1-46. Epub 2002 Mar 1. Ont Health Technol Assess Ser. 2002. PMID: 23074438 Free PMC article.
-
Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation.Circ Res. 2015 Feb 13;116(4):600-11. doi: 10.1161/CIRCRESAHA.116.304899. Epub 2015 Jan 6. Circ Res. 2015. PMID: 25563840 Free PMC article.
-
Comparison of cell-type-specific vs transmural aortic gene expression in experimental aneurysms.J Vasc Surg. 2005 May;41(5):844-52. doi: 10.1016/j.jvs.2005.02.027. J Vasc Surg. 2005. PMID: 15886670
-
Pathogenic and Therapeutic Significance of Angiotensin II Type I Receptor in Abdominal Aortic Aneurysms.Curr Drug Targets. 2018;19(11):1318-1326. doi: 10.2174/1389450119666180122155642. Curr Drug Targets. 2018. PMID: 29359665 Review.
-
Endoplasmic reticulum stress in abdominal aortic aneurysm.Int J Cardiol Heart Vasc. 2024 Aug 29;54:101500. doi: 10.1016/j.ijcha.2024.101500. eCollection 2024 Oct. Int J Cardiol Heart Vasc. 2024. PMID: 39280692 Free PMC article. Review.
Cited by
-
The role of 6-phosphogluconate dehydrogenase in vascular smooth muscle cell phenotypic switching and angioplasty-induced intimal hyperplasia.JVS Vasc Sci. 2024 Aug 2;5:100214. doi: 10.1016/j.jvssci.2024.100214. eCollection 2024. JVS Vasc Sci. 2024. PMID: 39318609 Free PMC article.
-
ER stress mediates Angiotensin II-augmented innate immunity memory and facilitates distinct susceptibilities of thoracic from abdominal aorta to aneurysm development.Front Immunol. 2023 Sep 4;14:1268916. doi: 10.3389/fimmu.2023.1268916. eCollection 2023. Front Immunol. 2023. PMID: 37731512 Free PMC article.
References
-
- Baxter B.T., Matsumura J., Curci J., et al. Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): design of a Phase IIb, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemp. Clin. Trials. May 2016;48:91–98. doi: 10.1016/j.cct.2016.03.008. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

