Novel, thalidomide-like, non-cereblon binding drug tetrafluorobornylphthalimide mitigates inflammation and brain injury
- PMID: 36872339
- PMCID: PMC9987061
- DOI: 10.1186/s12929-023-00907-5
Novel, thalidomide-like, non-cereblon binding drug tetrafluorobornylphthalimide mitigates inflammation and brain injury
Abstract
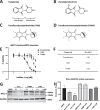
Background: Quelling microglial-induced excessive neuroinflammation is a potential treatment strategy across neurological disorders, including traumatic brain injury (TBI), and can be achieved by thalidomide-like drugs albeit this approved drug class is compromised by potential teratogenicity. Tetrafluorobornylphthalimide (TFBP) and tetrafluoronorbornylphthalimide (TFNBP) were generated to retain the core phthalimide structure of thalidomide immunomodulatory imide drug (IMiD) class. However, the classical glutarimide ring was replaced by a bridged ring structure. TFBP/TFNBP were hence designed to retain beneficial anti-inflammatory properties of IMiDs but, importantly, hinder cereblon binding that underlies the adverse action of thalidomide-like drugs.
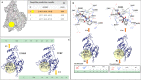
Methods: TFBP/TFNBP were synthesized and evaluated for cereblon binding and anti-inflammatory actions in human and rodent cell cultures. Teratogenic potential was assessed in chicken embryos, and in vivo anti-inflammatory actions in rodents challenged with either lipopolysaccharide (LPS) or controlled cortical impact (CCI) moderate traumatic brain injury (TBI). Molecular modeling was performed to provide insight into drug/cereblon binding interactions.
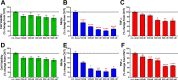
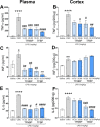
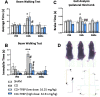
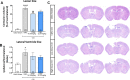
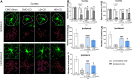
Results: TFBP/TFNBP reduced markers of inflammation in mouse macrophage-like RAW264.7 cell cultures and in rodents challenged with LPS, lowering proinflammatory cytokines. Binding studies demonstrated minimal interaction with cereblon, with no resulting degradation of teratogenicity-associated transcription factor SALL4 or of teratogenicity in chicken embryo assays. To evaluate the biological relevance of its anti-inflammatory actions, two doses of TFBP were administered to mice at 1 and 24 h post-injury following CCI TBI. Compared to vehicle treatment, TFBP reduced TBI lesion size together with TBI-induction of an activated microglial phenotype, as evaluated by immunohistochemistry 2-weeks post-injury. Behavioral evaluations at 1- and 2-weeks post-injury demonstrated TFBP provided more rapid recovery of TBI-induced motor coordination and balance impairments, versus vehicle treated mice.
Conclusion: TFBP and TFNBP represent a new class of thalidomide-like IMiDs that lower proinflammatory cytokine generation but lack binding to cereblon, the main teratogenicity-associated mechanism. This aspect makes TFBP and TFNBP potentially safer than classic IMiDs for clinical use. TFBP provides a strategy to mitigate excessive neuroinflammation associated with moderate severity TBI to, thereby, improve behavioral outcome measures and warrants further investigation in neurological disorders involving a neuroinflammatory component.
Keywords: Cereblon; Immunomodulatory imide drugs (IMiDs); Microglia; Neurodegeneration; Neuroinflammation; Spalt like transcription factor 4 (SALL4); Teratogenicity; Thalidomide.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
TFBP and TFNBP are protected under US Patent Application No. 63/397,235 2022. NHG, WL, DL, DT are coinventors and have assigned their rights in entirety to NIA, NIH (i.e., the US Government). DSK is, likewise, a co-inventor and has assigned his rights to AevisBio Inc. NIA, NIH and AevisBio Inc., have a Cooperative Research and Development Agreement to develop novel thalidomide-like drugs for the treatment of neurological disorders involving excessive inflammation. DSK, YKK, IH, SK are employees and shareholders of AevisBio Inc. All other authors declare no conflict of interest. The funders had no role in the design of the studies; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
A New Generation of IMiDs as Treatments for Neuroinflammatory and Neurodegenerative Disorders.Biomolecules. 2023 Apr 26;13(5):747. doi: 10.3390/biom13050747. Biomolecules. 2023. PMID: 37238617 Free PMC article. Review.
-
Activity of a Novel Anti-Inflammatory Agent F-3,6'-dithiopomalidomide as a Treatment for Traumatic Brain Injury.Biomedicines. 2022 Sep 30;10(10):2449. doi: 10.3390/biomedicines10102449. Biomedicines. 2022. PMID: 36289711 Free PMC article.
-
N-Adamantyl Phthalimidine: A New Thalidomide-like Drug That Lacks Cereblon Binding and Mitigates Neuronal and Synaptic Loss, Neuroinflammation, and Behavioral Deficits in Traumatic Brain Injury and LPS Challenge.ACS Pharmacol Transl Sci. 2021 Mar 30;4(2):980-1000. doi: 10.1021/acsptsci.1c00042. eCollection 2021 Apr 9. ACS Pharmacol Transl Sci. 2021. PMID: 33860215 Free PMC article.
-
Pomalidomide mitigates neuronal loss, neuroinflammation, and behavioral impairments induced by traumatic brain injury in rat.J Neuroinflammation. 2016 Jun 28;13(1):168. doi: 10.1186/s12974-016-0631-6. J Neuroinflammation. 2016. PMID: 27353053 Free PMC article.
-
Neuroinflammation as a Factor of Neurodegenerative Disease: Thalidomide Analogs as Treatments.Front Cell Dev Biol. 2019 Dec 4;7:313. doi: 10.3389/fcell.2019.00313. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31867326 Free PMC article. Review.
Cited by
-
A New Generation of IMiDs as Treatments for Neuroinflammatory and Neurodegenerative Disorders.Biomolecules. 2023 Apr 26;13(5):747. doi: 10.3390/biom13050747. Biomolecules. 2023. PMID: 37238617 Free PMC article. Review.
-
2-(Piperidin-3-yl)phthalimides reduce classical markers of cellular inflammation in LPS-challenged RAW 264.7 cells and also demonstrate potentially relevant sigma and serotonin receptor affinity in membrane preparations.Bioorg Med Chem Lett. 2024 Sep 15;110:129885. doi: 10.1016/j.bmcl.2024.129885. Epub 2024 Jul 10. Bioorg Med Chem Lett. 2024. PMID: 38996940
-
Zinc oxide nanoparticles induces cell death and consequently leading to incomplete neural tube closure through oxidative stress during embryogenesis.Cell Biol Toxicol. 2024 Jul 3;40(1):51. doi: 10.1007/s10565-024-09894-1. Cell Biol Toxicol. 2024. PMID: 38958792 Free PMC article.
-
Thalidomide upper limb embryopathy - pathogenesis, past and present management and future considerations.J Hand Surg Eur Vol. 2023 Sep;48(8):699-709. doi: 10.1177/17531934231177425. Epub 2023 May 24. J Hand Surg Eur Vol. 2023. PMID: 37226469 Free PMC article. Review.
-
Assessment of the Teratogenic Effect of Drugs on the Chicken Embryo.Methods Mol Biol. 2024;2753:251-260. doi: 10.1007/978-1-0716-3625-1_12. Methods Mol Biol. 2024. PMID: 38285343
References
-
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic Brain Injury in the civilian population of the United Statet 2005. J Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.AC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

