Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review
- PMID: 36865479
- PMCID: PMC9970931
- DOI: 10.1016/j.heliyon.2023.e13464
Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review
Abstract
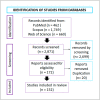
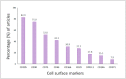
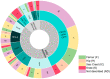
Multipotent mesenchymal stromal cells (MSCs) have been described as bone marrow stromal cells, which can form cartilage, bone or hematopoietic supportive stroma. In 2006, the International Society for Cell Therapy (ISCT) established a set of minimal characteristics to define MSCs. According to their criteria, these cells must express CD73, CD90 and CD105 surface markers; however, it is now known they do not represent true stemness epitopes. The objective of the present work was to determine the surface markers for human MSCs associated with skeletal tissue reported in the literature (1994-2021). To this end, we performed a scoping review for hMSCs in axial and appendicular skeleton. Our findings determined the most widely used markers were CD105 (82.9%), CD90 (75.0%) and CD73 (52.0%) for studies performed in vitro as proposed by the ISCT, followed by CD44 (42.1%), CD166 (30.9%), CD29 (27.6%), STRO-1 (17.7%), CD146 (15.1%) and CD271 (7.9%) in bone marrow and cartilage. On the other hand, only 4% of the articles evaluated in situ cell surface markers. Even though most studies use the ISCT criteria, most publications in adult tissues don't evaluate the characteristics that establish a stem cell (self-renewal and differentiation), which will be necessary to distinguish between a stem cell and progenitor populations. Collectively, MSCs require further understanding of their characteristics if they are intended for clinical use.
Keywords: Bone; Bone marrow; Cartilage; Cell surface marker; Mesenchymal stem cell (MSCs).
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production.Stem Cell Res Ther. 2016 Aug 11;7(1):107. doi: 10.1186/s13287-016-0370-8. Stem Cell Res Ther. 2016. PMID: 27515308 Free PMC article.
-
Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection.Bone. 2012 Mar;50(3):804-10. doi: 10.1016/j.bone.2011.12.014. Epub 2011 Dec 28. Bone. 2012. PMID: 22226689 Free PMC article.
-
ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation.Gene X. 2020 Mar 14;5:100031. doi: 10.1016/j.gene.2020.100031. eCollection 2020 Dec. Gene X. 2020. PMID: 32550557 Free PMC article.
-
Concise review: the surface markers and identity of human mesenchymal stem cells.Stem Cells. 2014 Jun;32(6):1408-19. doi: 10.1002/stem.1681. Stem Cells. 2014. PMID: 24578244 Review.
-
Mesenchymal stem cell markers in periodontal tissues and periapical lesions.Acta Histochem. 2020 Dec;122(8):151636. doi: 10.1016/j.acthis.2020.151636. Epub 2020 Oct 22. Acta Histochem. 2020. PMID: 33132168 Review.
Cited by
-
Multipotent mesenchymal stromal/stem cell-based therapies for acute respiratory distress syndrome: current progress, challenges, and future frontiers.Braz J Med Biol Res. 2024 Oct 14;57:e13219. doi: 10.1590/1414-431X2024e13219. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 39417447 Free PMC article. Review.
-
Mesenchymal Stem Cells in Soft Tissue Regenerative Medicine: A Comprehensive Review.Medicina (Kaunas). 2023 Aug 10;59(8):1449. doi: 10.3390/medicina59081449. Medicina (Kaunas). 2023. PMID: 37629738 Free PMC article. Review.
-
Application of polydopamine-modified triphasic PLA/PCL-PLGA/Mg(OH)2-velvet antler polypeptides scaffold loaded with fibrocartilage stem cells for the repair of osteochondral defects.Front Bioeng Biotechnol. 2024 Sep 19;12:1460623. doi: 10.3389/fbioe.2024.1460623. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39372430 Free PMC article.
-
Liraglutide attenuates obese-associated breast cancer cell proliferation via inhibiting PI3K/Akt/mTOR signaling pathway.Saudi Pharm J. 2024 Jan;32(1):101923. doi: 10.1016/j.jsps.2023.101923. Epub 2023 Dec 18. Saudi Pharm J. 2024. PMID: 38223522 Free PMC article.
-
Bone mesenchymal stem cells improve cholestatic liver fibrosis by targeting ULK1 to regulate autophagy through PI3K/AKT/mTOR pathway.Stem Cells Transl Med. 2024 Jul 15;13(7):648-660. doi: 10.1093/stcltm/szae028. Stem Cells Transl Med. 2024. PMID: 38736295 Free PMC article.
References
-
- Friedenstein A.J., Piatetzky-Shapiro, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. Development. 1966;16(3):381–390. Dec 1. - PubMed
-
- Owen M., Friedenstein A.J. Ciba Foundation Symposium. John Wiley & Sons, Ltd; 1988. Stromal stem cells: marrow-derived osteogenic precursors; pp. 42–60. - PubMed
-
- Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9(5):641–650. Sep. 1. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

