Platelets Facilitate Wound Healing by Mitochondrial Transfer and Reducing Oxidative Stress in Endothelial Cells
- PMID: 36860732
- PMCID: PMC9970712
- DOI: 10.1155/2023/2345279
Platelets Facilitate Wound Healing by Mitochondrial Transfer and Reducing Oxidative Stress in Endothelial Cells
Abstract
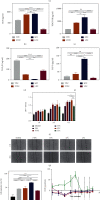
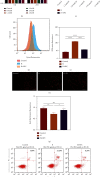
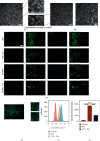
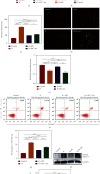
As a critical member in wound healing, vascular endothelial cells (ECs) impaired under high levels of reactive oxygen species (ROS) would hamper neovascularization. Mitochondria transfer can reduce intracellular ROS damage under pathological condition. Meanwhile, platelets can release mitochondria and alleviate oxidative stress. However, the mechanism by which platelets promote cell survival and reduce oxidative stress damage has not been clarified. Here, first, we selected ultrasound as the best method for subsequent experiments by detecting the growth factors and mitochondria released from manipulation platelet concentrates (PCs), as well as the effect of manipulation PCs on the proliferation and migration of HUVECs. Then, we found that sonicate platelet concentrates (SPC) decreased the level of ROS in HUVECs treated with hydrogen peroxide in advance, increased mitochondrial membrane potential, and reduced apoptosis. By transmission electron microscope, we saw that two kinds of mitochondria, free or wrapped in vesicles, were released by activated platelets. In addition, we explored that platelet-derived mitochondria were transferred to HUVECs partly by means of dynamin-dependent clathrin-mediated endocytosis. Consistently, we determined that platelet-derived mitochondria reduced apoptosis of HUVECs caused by oxidative stress. What is more, we screened survivin as the target of platelet-derived mitochondria via high-throughput sequencing. Finally, we demonstrated that platelet-derived mitochondria promoted wound healing in vivo. Overall, these findings revealed that platelets are important donors of mitochondria, and platelet-derived mitochondria can promote wound healing by reducing apoptosis caused by oxidative stress in vascular endothelial cells. And survivin is a potential target. These results further expand the knowledge of the platelet function and provide new insights into the role of platelet-derived mitochondria in wound healing.
Copyright © 2023 Panshi Jin et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts.Platelets. 2022 Dec;34(1):2151996. doi: 10.1080/09537104.2022.2151996. Platelets. 2022. PMID: 36529914
-
Platelet-derived respiratory-competent mitochondria transfer to mesenchymal stem cells to promote wound healing via metabolic reprogramming.Platelets. 2022 Feb 17;33(2):171-173. doi: 10.1080/09537104.2021.1961717. Epub 2022 Feb 3. Platelets. 2022. PMID: 35112646
-
Exogenous hydrogen sulphide supplement accelerates skin wound healing via oxidative stress inhibition and vascular endothelial growth factor enhancement.Exp Dermatol. 2019 Jul;28(7):776-785. doi: 10.1111/exd.13930. Epub 2019 May 15. Exp Dermatol. 2019. PMID: 30927279
-
The initiation of oxidative stress and therapeutic strategies in wound healing.Biomed Pharmacother. 2023 Jan;157:114004. doi: 10.1016/j.biopha.2022.114004. Epub 2022 Nov 11. Biomed Pharmacother. 2023. PMID: 36375308 Review.
-
Cross-Talk between NADPH Oxidase and Mitochondria: Role in ROS Signaling and Angiogenesis.Cells. 2020 Aug 6;9(8):1849. doi: 10.3390/cells9081849. Cells. 2020. PMID: 32781794 Free PMC article. Review.
Cited by
-
Mitochondrial transplantation: a promising strategy for treating degenerative joint diseases.J Transl Med. 2024 Oct 15;22(1):941. doi: 10.1186/s12967-024-05752-0. J Transl Med. 2024. PMID: 39407249 Free PMC article. Review.
-
Progress in Pluronic F127 Derivatives for Application in Wound Healing and Repair.Int J Nanomedicine. 2023 Aug 7;18:4485-4505. doi: 10.2147/IJN.S418534. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37576462 Free PMC article. Review.
-
From dysfunction to healing: advances in mitochondrial therapy for Osteoarthritis.J Transl Med. 2024 Nov 11;22(1):1013. doi: 10.1186/s12967-024-05799-z. J Transl Med. 2024. PMID: 39529128 Free PMC article. Review.
-
Enhanced osteogenic differentiation in 3D hydrogel scaffold via macrophage mitochondrial transfer.J Nanobiotechnology. 2024 Sep 5;22(1):540. doi: 10.1186/s12951-024-02757-1. J Nanobiotechnology. 2024. PMID: 39237942 Free PMC article.
-
Pharmacotherapy for Keloids and Hypertrophic Scars.Int J Mol Sci. 2024 Apr 25;25(9):4674. doi: 10.3390/ijms25094674. Int J Mol Sci. 2024. PMID: 38731893 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

