Heparin-based sericin hydrogel-encapsulated basic fibroblast growth factor for in vitro and in vivo skin repair
- PMID: 36851964
- PMCID: PMC9958445
- DOI: 10.1016/j.heliyon.2023.e13554
Heparin-based sericin hydrogel-encapsulated basic fibroblast growth factor for in vitro and in vivo skin repair
Abstract
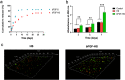
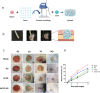
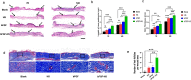
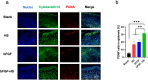
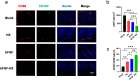
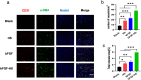
The treatment of full-thickness cutaneous wounds remains a significant challenge in clinical therapeutics. Exogenous growth factor (GF) has been applied in clinics to promote wound healing. However, the retention of GF on the wound bed after its direct application to the wound surface is difficult. Moreover, growth factors (GFs) are always inactivated in the complex wound healing microenvironment due to various factors, which significantly decrease the therapeutic effect. Sericin hydrogel (S) can be used as an effective carrier for GFs owing to its low immunogenicity, good biocompatibility, and good healing-promoting ability. Here, we designed a heparin-based sericin hydrogel (HS) -encapsulated basic fibroblast growth factor (bFGF-HS) to facilitate wound healing and skin regeneration. The hydrogel exhibited a three-dimensional (3D) microporous structure, excellent biodegradability, good adhesiveness, and low cytotoxicity. In vitro release of bFGF from bFGF-HS coacervates revealed that bFGF-HS might control the release of bFGF within 25 days through heparin regulation. bFGF-HS significantly promoted vascularization and re-epithelialization and improved collagen deposition, ultimately accelerating wound healing in vivo in mice. bFGF-HS treated wounds were also found to have more hair follicles and milder inflammatory reactions. Overall, this study provides a new therapeutic approach for full-thickness skin defect wounds using bFGF-HS.
Keywords: Sericin hydrogel; Wound healin; bFGF.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Comparative Study of Heparin-Poloxamer Hydrogel Modified bFGF and aFGF for in Vivo Wound Healing Efficiency.ACS Appl Mater Interfaces. 2016 Jul 27;8(29):18710-21. doi: 10.1021/acsami.6b06047. Epub 2016 Jul 15. ACS Appl Mater Interfaces. 2016. PMID: 27384134
-
Bioinspired Collagen Scaffold Loaded with bFGF-Overexpressing Human Mesenchymal Stromal Cells Accelerating Diabetic Skin Wound Healing via HIF-1 Signal Pathway Regulated Neovascularization.ACS Appl Mater Interfaces. 2024 Sep 4;16(35):45989-46004. doi: 10.1021/acsami.4c08174. Epub 2024 Aug 21. ACS Appl Mater Interfaces. 2024. PMID: 39165237
-
Acceleration of skin regeneration in full-thickness burns by incorporation of bFGF-loaded alginate microspheres into a CMCS-PVA hydrogel.J Tissue Eng Regen Med. 2017 May;11(5):1562-1573. doi: 10.1002/term.2057. Epub 2015 Jun 29. J Tissue Eng Regen Med. 2017. PMID: 26118827
-
Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy.Int J Nanomedicine. 2019 Jul 18;14:5449-5475. doi: 10.2147/IJN.S213883. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31409998 Free PMC article.
-
Bioactive ECM Mimic Hyaluronic Acid Dressing via Sustained Releasing of bFGF for Enhancing Skin Wound Healing.ACS Appl Bio Mater. 2020 May 18;3(5):3039-3048. doi: 10.1021/acsabm.0c00096. Epub 2020 Apr 13. ACS Appl Bio Mater. 2020. PMID: 35025350
Cited by
-
Fabrication and evaluation of a bi-layered electrospun PCL/PVA patch for wound healing: Release of vitamins and silver nanoparticle.Heliyon. 2024 Jun 15;10(12):e33178. doi: 10.1016/j.heliyon.2024.e33178. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38994056 Free PMC article.
-
Enhancing Wound Healing: A Comprehensive Review of Sericin and Chelidonium majus L. as Potential Dressings.Materials (Basel). 2024 Aug 24;17(17):4199. doi: 10.3390/ma17174199. Materials (Basel). 2024. PMID: 39274589 Free PMC article. Review.
-
Protein-modified nanomaterials: emerging trends in skin wound healing.Discov Nano. 2023 Oct 16;18(1):127. doi: 10.1186/s11671-023-03903-8. Discov Nano. 2023. PMID: 37843732 Free PMC article. Review.
-
Facile fabrication and biological investigations of metal oxides intercalated in kaolinite clay-based dressing material to improve wound healing ability in nursing care after post-operative period.Heliyon. 2024 Jan 26;10(3):e25289. doi: 10.1016/j.heliyon.2024.e25289. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38333785 Free PMC article.
References
-
- Kim H.S., Sun X., Lee J.H., Kim H.W., Fu X., Leong K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019;146:209–239. - PubMed
-
- Morton L.M., Phillips T.J. Wound healing and treating wounds: differential diagnosis and evaluation of chronic wounds. J. Am. Acad. Dermatol. 2016;74(4):589–605. quiz 605-6. - PubMed
-
- Nosrati H., Khodaei M., Alizadeh Z., Banitalebi-Dehkordi M. Cationic, anionic and neutral polysaccharides for skin tissue engineering and wound healing applications. Int. J. Biol. Macromol. 2021;192:298–322. - PubMed
-
- Zhao L., Zhao J., Zhang F., Xu Z., Chen F., Shi Y., Hou C., Huang Y., Lin C., Yu R., Guo W. Highly stretchable, adhesive, and self-healing silk fibroin-dopted hydrogels for wearable sensors. Adv Healthc Mater. 2021;10(14) - PubMed
-
- El-Ashram S., El-Samad L.M., Basha A.A., El Wakil A. Naturally-derived targeted therapy for wound healing: beyond classical strategies. Pharmacol. Res. 2021;170 - PubMed
LinkOut - more resources
Full Text Sources

