Hemorrhagic Fever with Renal Syndrome in Asia: History, Pathogenesis, Diagnosis, Treatment, and Prevention
- PMID: 36851775
- PMCID: PMC9966805
- DOI: 10.3390/v15020561
Hemorrhagic Fever with Renal Syndrome in Asia: History, Pathogenesis, Diagnosis, Treatment, and Prevention
Abstract
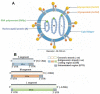
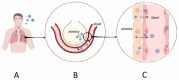
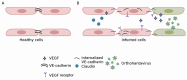
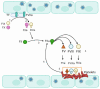
Hemorrhagic Fever with Renal Syndrome (HFRS) is the most frequently diagnosed zoonosis in Asia. This zoonotic infection is the result of exposure to the virus-contaminated aerosols. Orthohantavirus infection may cause Hemorrhagic Fever with Renal Syndrome (HRFS), a disease that is characterized by acute kidney injury and increased vascular permeability. Several species of orthohantaviruses were identified as causing infection, where Hantaan, Puumala, and Seoul viruses are most common. Orthohantaviruses are endemic to several Asian countries, such as China, South Korea, and Japan. Along with those countries, HFRS tops the list of zoonotic infections in the Far Eastern Federal District of Russia. Recently, orthohantavirus circulation was demonstrated in small mammals in Thailand and India, where orthohantavirus was not believed to be endemic. In this review, we summarized the current data on orthohantaviruses in Asia. We gave the synopsis of the history and diversity of orthohantaviruses in Asia. We also described the clinical presentation and current understanding of the pathogenesis of orthohantavirus infection. Additionally, conventional and novel approaches for preventing and treating orthohantavirus infection are discussed.
Keywords: emerging viruses; immunology; orthohantaviruses; therapeutics; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Haemorrhagic fever with renal syndrome, virological and epidemiological aspects.Pediatr Nephrol. 1992 Mar;6(2):201-4. doi: 10.1007/BF00866318. Pediatr Nephrol. 1992. PMID: 1349231 Review.
-
Cases of Hemorrhagic Fever with Renal Syndrome in Russia during 2000-2022.Viruses. 2023 Jul 12;15(7):1537. doi: 10.3390/v15071537. Viruses. 2023. PMID: 37515224 Free PMC article. Review.
-
Haemorrhagic fever with renal syndrome: literature review and distribution analysis in China.Int J Infect Dis. 2016 Feb;43:95-100. doi: 10.1016/j.ijid.2016.01.003. Epub 2016 Jan 11. Int J Infect Dis. 2016. PMID: 26791541 Review.
-
Cells of the human respiratory tract support the replication of pathogenic Old World orthohantavirus Puumala.Virol J. 2021 Aug 17;18(1):169. doi: 10.1186/s12985-021-01636-7. Virol J. 2021. PMID: 34404450 Free PMC article.
-
Hantavirus infections in humans and animals, China.Emerg Infect Dis. 2010 Aug;16(8):1195-203. doi: 10.3201/eid1608.090470. Emerg Infect Dis. 2010. PMID: 20678311 Free PMC article.
Cited by
-
A retrospective study of variations in the kinds of diseases discharged from the Department of Infectious Diseases of a large general hospital in Central China during 2013-2019.Front Public Health. 2024 Feb 14;12:1289972. doi: 10.3389/fpubh.2024.1289972. eCollection 2024. Front Public Health. 2024. PMID: 38420029 Free PMC article.
-
A bibliometric analysis of domestic and international research on hemorrhagic fever with renal syndrome over the past 2 decades.Medicine (Baltimore). 2024 Sep 13;103(37):e39737. doi: 10.1097/MD.0000000000039737. Medicine (Baltimore). 2024. PMID: 39287241 Free PMC article.
-
Asymmetric impact of climatic parameters on hemorrhagic fever with renal syndrome in Shandong using a nonlinear autoregressive distributed lag model.Sci Rep. 2024 Apr 28;14(1):9739. doi: 10.1038/s41598-024-58023-9. Sci Rep. 2024. PMID: 38679612 Free PMC article.
-
Epidemiological and clinical characteristics of death from hemorrhagic fever with renal syndrome: a meta-analysis.Front Microbiol. 2024 Apr 4;15:1329683. doi: 10.3389/fmicb.2024.1329683. eCollection 2024. Front Microbiol. 2024. PMID: 38638893 Free PMC article.
-
A comprehensive investigation of Glycoprotein-based nucleic acid vaccines for Hantaan Virus.NPJ Vaccines. 2024 Oct 23;9(1):196. doi: 10.1038/s41541-024-00991-0. NPJ Vaccines. 2024. PMID: 39443512 Free PMC article.
References
-
- ICTV Current ICTV Taxonomy Release. [(accessed on 8 August 2022)]. Available online: https://ictv.global/taxonomy.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

