Comprehensive Profiling of EBV Gene Expression and Promoter Methylation Reveals Latency II Viral Infection and Sporadic Abortive Lytic Activation in Peripheral T-Cell Lymphomas
- PMID: 36851637
- PMCID: PMC9960980
- DOI: 10.3390/v15020423
Comprehensive Profiling of EBV Gene Expression and Promoter Methylation Reveals Latency II Viral Infection and Sporadic Abortive Lytic Activation in Peripheral T-Cell Lymphomas
Abstract
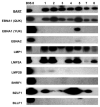
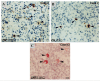
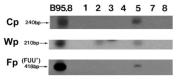
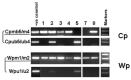
Epstein-Barr virus (EBV) latency patterns are well defined in EBV-associated epithelial, NK/T-cell, and B-cell malignancies, with links between latency stage and tumorigenesis deciphered in various studies. In vitro studies suggest that the oncogenic activity of EBV in T-cells might be somewhat different from that in EBV-tropic B lymphoid cells, prompting us to study this much less investigated viral gene expression pattern and its regulation in nine EBV+ peripheral T-cell lymphoma (PTCL) biopsies. Using frozen specimens, RT-PCR showed 6/7 cases with a latency II pattern of EBV gene expression. Analyses of EBNA1 promoter usage and CpG methylation status in these six cases showed that only Qp was used, while Cp, Wp, and Fp were all silent. However, the remaining case showed an exceptionally unique latency III type with lytic activation, as evidenced by EBV lytic clonality and confirmed by the full usage of Cp and Qp as well as weakly lytic Fp and Wp, fully unmethylated Cp and marginally unmethylated Wp. Further immunostaining of the eight cases revealed a few focally clustered LMP1+ cells in 7/8 cases, with rare isolated LMP1+ cells detected in another case. Double immunostaining confirmed that the LMP1+ cells were of the T-cell phenotype (CD3+). In 6/8 cases, sporadically scattered Zta+ cells were detected. Double staining of EBER-ISH with T-cell (CD45RO/UCHL1) or B-cell (CD20) markers confirmed that the vast majority of EBER+ cells were of the T-cell phenotype. Predominant type-A EBV variant and LMP1 30-bp deletion variant were present, with both F and f variants detected. In summary, the EBV gene expression pattern in PTCL was found to be mainly of latency II (BART+EBNA1(Qp)+LMP1+LMP2A+BZLF1+), similar to that previously reported in EBV-infected nasopharyngeal epithelial, NK/T-cell, and Hodgkin malignancies; however, fully lytic infection could also be detected in occasional cases. Rare cells with sporadic immediate-early gene expression were commonly detected in PTCL. These findings have implications for the future development of EBV-targeting therapeutics for this cancer.
Keywords: CpG methylation; EBV; LMP1; Zta; latency; peripheral T-cell lymphoma; promoter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Latency pattern of Epstein-Barr virus and methylation status in Epstein-Barr virus-associated hemophagocytic syndrome.J Med Virol. 2003 Jul;70(3):410-9. doi: 10.1002/jmv.10411. J Med Virol. 2003. PMID: 12767005
-
Epstein-Barr virus mRNA profiles and viral DNA methylation status in nasopharyngeal brushings from nasopharyngeal carcinoma patients reflect tumor origin.Int J Cancer. 2017 Jan 1;140(1):149-162. doi: 10.1002/ijc.30418. Epub 2016 Sep 23. Int J Cancer. 2017. PMID: 27600027 Free PMC article.
-
Epstein-Barr virus infection and its gene expression in gastric lymphoma of mucosa-associated lymphoid tissue.J Med Virol. 1998 Dec;56(4):342-50. doi: 10.1002/(sici)1096-9071(199812)56:4<342::aid-jmv10>3.0.co;2-p. J Med Virol. 1998. PMID: 9829640
-
Host cell-dependent expression of latent Epstein-Barr virus genomes: regulation by DNA methylation.Adv Cancer Res. 2003;89:133-56. doi: 10.1016/s0065-230x(03)01004-2. Adv Cancer Res. 2003. PMID: 14587872 Review.
-
Targeting latent viral infection in EBV-associated lymphomas.Front Immunol. 2024 Feb 23;15:1342455. doi: 10.3389/fimmu.2024.1342455. eCollection 2024. Front Immunol. 2024. PMID: 38464537 Free PMC article. Review.
Cited by
-
Cancer and HIV: The Molecular Mechanisms of the Deadly Duo.Cancers (Basel). 2024 Jan 26;16(3):546. doi: 10.3390/cancers16030546. Cancers (Basel). 2024. PMID: 38339297 Free PMC article. Review.
-
Contribution of the Epstein-Barr virus to the oncogenesis of mature T-cell lymphoproliferative neoplasms.Front Oncol. 2023 Sep 14;13:1240359. doi: 10.3389/fonc.2023.1240359. eCollection 2023. Front Oncol. 2023. PMID: 37781191 Free PMC article. Review.
-
Cotargeting EBV lytic as well as latent cycle antigens increases T-cell potency against lymphoma.Blood Adv. 2024 Jul 9;8(13):3360-3371. doi: 10.1182/bloodadvances.2023012183. Blood Adv. 2024. PMID: 38640255 Free PMC article.
-
Characterization of latently infected EBV+ antibody-secreting B cells isolated from ovarian tumors and malignant ascites.Front Immunol. 2024 Jul 17;15:1379175. doi: 10.3389/fimmu.2024.1379175. eCollection 2024. Front Immunol. 2024. PMID: 39086481 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

