Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection
- PMID: 36851578
- PMCID: PMC9965858
- DOI: 10.3390/v15020364
Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection
Abstract
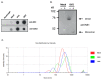
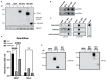
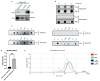
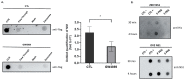
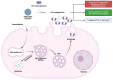
Extracellular vesicles (EVs), produced during viral infections, are of emerging interest in understanding infectious processes and host-pathogen interactions. EVs and exosomes in particular have the natural ability to transport nucleic acids, proteins, and other components of cellular or viral origin. Thus, they participate in intercellular communication, immune responses, and infectious and pathophysiological processes. Some viruses are known to hijack the cell production and content of EVs for their benefit. Here, we investigate whether two pathogenic flaviviruses i.e., Zika Virus (ZIKV) and Dengue virus (DENV2) could have an impact on the features of EVs. The analysis of EVs produced by infected cells allowed us to identify that the non-structural protein 1 (NS1), described as a viral toxin, is associated with exosomes. This observation could be confirmed under conditions of overexpression of recombinant NS1 from each flavivirus. Using different isolation methods (i.e., exosome isolation kit, size exclusion chromatography, Polyethylene Glycol enrichment, and ELISA capture), we showed that NS1 was present as a dimer at the surface of excreted exosomes, and that this association could occur in the extracellular compartment. This finding could be of major importance in a physiological context. Indeed, this capacity of NS1 to address EVs and its implication in the pathophysiology during Dengue or Zika diseases should be explored. Furthermore, exosomes that have demonstrated a natural capacity to vectorize NS1 could serve as useful tools for vaccine development.
Keywords: Dengue; NS1; Zika; exosome; flavivirus; viral toxin vectorization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Delayed and highly specific antibody response to nonstructural protein 1 (NS1) revealed during natural human ZIKV infection by NS1-based capture ELISA.BMC Infect Dis. 2018 Jun 14;18(1):275. doi: 10.1186/s12879-018-3173-y. BMC Infect Dis. 2018. PMID: 29898684 Free PMC article.
-
The Dengue Virus Nonstructural Protein 1 (NS1) Is Secreted from Mosquito Cells in Association with the Intracellular Cholesterol Transporter Chaperone Caveolin Complex.J Virol. 2019 Feb 5;93(4):e01985-18. doi: 10.1128/JVI.01985-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463973 Free PMC article.
-
Combination of Nonstructural Protein 1-Based Enzyme-Linked Immunosorbent Assays Can Detect and Distinguish Various Dengue Virus and Zika Virus Infections.J Clin Microbiol. 2019 Jan 30;57(2):e01464-18. doi: 10.1128/JCM.01464-18. Print 2019 Feb. J Clin Microbiol. 2019. PMID: 30429254 Free PMC article.
-
The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell-Cell Communication: New Insights on Virus-Host Interactions.Viruses. 2020 Jul 16;12(7):765. doi: 10.3390/v12070765. Viruses. 2020. PMID: 32708685 Free PMC article. Review.
-
The Dual Role of the Antibody Response Against the Flavivirus Non-structural Protein 1 (NS1) in Protection and Immuno-Pathogenesis.Front Immunol. 2019 Jul 18;10:1651. doi: 10.3389/fimmu.2019.01651. eCollection 2019. Front Immunol. 2019. PMID: 31379848 Free PMC article. Review.
Cited by
-
The biology and function of extracellular vesicles in immune response and immunity.Immunity. 2024 Aug 13;57(8):1752-1768. doi: 10.1016/j.immuni.2024.07.009. Immunity. 2024. PMID: 39142276 Review.
-
Control of maternal Zika virus infection during pregnancy is associated with lower antibody titers in a macaque model.Front Immunol. 2023 Sep 22;14:1267638. doi: 10.3389/fimmu.2023.1267638. eCollection 2023. Front Immunol. 2023. PMID: 37809089 Free PMC article.
-
Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation.Int J Mol Sci. 2024 Feb 10;25(4):2144. doi: 10.3390/ijms25042144. Int J Mol Sci. 2024. PMID: 38396820 Free PMC article. Review.
-
A Review Study of the Participation of Late Domains in Sorting and Transport of Viral Factors to Exosomes.Life (Basel). 2023 Aug 31;13(9):1842. doi: 10.3390/life13091842. Life (Basel). 2023. PMID: 37763246 Free PMC article. Review.
-
Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design.Vaccines (Basel). 2024 Mar 7;12(3):280. doi: 10.3390/vaccines12030280. Vaccines (Basel). 2024. PMID: 38543914 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

