Assessment of the Interferon-Lambda-3 Polymorphism in the Antibody Response to COVID-19 in Older Adults Seropositive for CMV
- PMID: 36851357
- PMCID: PMC9963200
- DOI: 10.3390/vaccines11020480
Assessment of the Interferon-Lambda-3 Polymorphism in the Antibody Response to COVID-19 in Older Adults Seropositive for CMV
Abstract
Background: Here, we investigated the impact of IFN-lambda-3 polymorphism on specific IgG responses for COVID-19 in older adults seropositive for CMV.
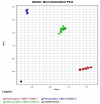
Methods: Blood samples of 25 older adults of both sexes were obtained at three different times: during a micro-outbreak (MO) of SARS-CoV-2 in 2020; eight months after (CURE); and 30 days after the administration of the second dose of ChadOx-1 vaccine (VAC). The specific IgG for both SARS-CoV-2 and CMV antigens, neutralizing antibodies against SARS-CoV-2, and also the polymorphism profile for IFN-lambda-3 (rs12979860 C > T) were assessed.
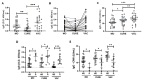
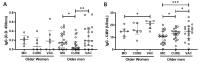
Results: Higher levels of specific IgG for SARS-CoV-2 antigens were found in the MO and VAC than in the CURE time-point. Volunteers with specific neutralizing antibodies against SARS-CoV-2 showed better specific IgG responses for SARS-CoV-2 and lower specific IgG levels for CMV than volunteers without specific neutralizing antibodies. Significant negative correlations between the specific IgG levels for SARS-CoV-2 and CMV were found at the MO time-point, as well as in the group of individuals homozygous for allele 1 (C/C) in the MO time-point and heterozygotes (C/T) in the CURE time-point.
Conclusion: Our results suggested that both CMV seropositivity and the homozygosis for allele 1 (C/C) in IFN-lambda-3 gene can negatively impact the antibody response to COVID-19 infection and vaccination in older adults.
Keywords: SARS-CoV-2; allele; cytomegalovirus; immunoglobulins; neutralizing antibody; outbreak.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Cytomegalovirus Seropositivity in Older Adults Changes the T Cell Repertoire but Does Not Prevent Antibody or Cellular Responses to SARS-CoV-2 Vaccination.J Immunol. 2022 Nov 15;209(10):1892-1905. doi: 10.4049/jimmunol.2200369. J Immunol. 2022. PMID: 36426948 Free PMC article.
-
Kinetics of SARS-CoV-2 Specific and Neutralizing Antibodies over Seven Months after Symptom Onset in COVID-19 Patients.Microbiol Spectr. 2021 Oct 31;9(2):e0059021. doi: 10.1128/Spectrum.00590-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550000 Free PMC article.
-
Evaluation of post-vaccination immunoglobulin G antibodies and T-cell immune response after inoculation with different types and doses of SARS-CoV-2 vaccines: A retrospective cohort study.Front Med (Lausanne). 2023 Jan 10;9:1092646. doi: 10.3389/fmed.2022.1092646. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36703898 Free PMC article.
-
Longitudinal profile of neutralizing and binding antibodies in vaccinated and convalescent COVID-19 cohorts by chemiluminescent immunoassays.Immun Inflamm Dis. 2022 Jun;10(6):e612. doi: 10.1002/iid3.612. Immun Inflamm Dis. 2022. PMID: 35634960 Free PMC article.
-
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days.Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554. eCollection 2021. Front Immunol. 2021. PMID: 35003104 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

