Development of a novel ex vivo organ culture system to improve preservation methods of regenerative tissues
- PMID: 36849572
- PMCID: PMC9971270
- DOI: 10.1038/s41598-023-29629-2
Development of a novel ex vivo organ culture system to improve preservation methods of regenerative tissues
Abstract
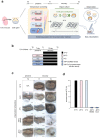
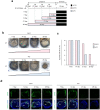
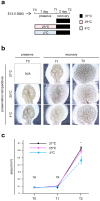
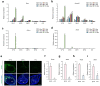
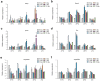
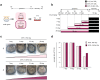
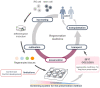
Recent advances in regenerative technology have made the regeneration of various organs using pluripotent stem cells possible. However, a simpler screening method for evaluating regenerated organs is required to apply this technology to clinical regenerative medicine in the future. We have developed a simple evaluation method using a mouse tooth germ culture model of organs formed by epithelial-mesenchymal interactions. In this study, we successfully established a simple method that controls tissue development in a temperature-dependent manner using a mouse tooth germ ex vivo culture model. We observed that the development of the cultured tooth germ could be delayed by low-temperature culture and resumed by the subsequent culture at 37 °C. Furthermore, the optimal temperature for the long-term preservation of tooth germ was 25 °C, a subnormothermic temperature that maintains the expression of stem cell markers. We also found that subnormothermic temperature induces the expression of cold shock proteins, such as cold-inducible RNA-binding protein, RNA-binding motif protein 3, and serine and arginine rich splicing factor 5. This study provides a simple screening method to help establish the development of regenerative tissue technology using a tooth organ culture model. Our findings may be potentially useful for making advances in the field of regenerative medicine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Whole Tooth Regeneration as a Future Dental Treatment.Adv Exp Med Biol. 2015;881:255-69. doi: 10.1007/978-3-319-22345-2_14. Adv Exp Med Biol. 2015. PMID: 26545754 Review.
-
Development and prospects of organ replacement regenerative therapy.Cornea. 2013 Nov;32 Suppl 1:S13-21. doi: 10.1097/ICO.0b013e3182a18e6c. Cornea. 2013. PMID: 24104927 Review.
-
H2S supplementation: A novel method for successful organ preservation at subnormothermic temperatures.Nitric Oxide. 2018 Dec 1;81:57-66. doi: 10.1016/j.niox.2018.10.004. Epub 2018 Oct 25. Nitric Oxide. 2018. PMID: 30393129
-
Mouse Embryonic Tooth Germ Dissection and Ex vivo Culture Protocol.Bio Protoc. 2020 Feb 5;10(3):e3515. doi: 10.21769/BioProtoc.3515. eCollection 2020 Feb 5. Bio Protoc. 2020. PMID: 33654740 Free PMC article.
-
The development of a bioengineered organ germ method.Nat Methods. 2007 Mar;4(3):227-30. doi: 10.1038/nmeth1012. Epub 2007 Feb 18. Nat Methods. 2007. PMID: 17322892
Cited by
-
Regeneration of amputated mice digit tips by including Wnt signaling pathway with CHIR99021 and IWP-2 chemicals in limb organ culture system.Iran J Basic Med Sci. 2024;27(10):1251-1259. doi: 10.22038/ijbms.2024.76957.16643. Iran J Basic Med Sci. 2024. PMID: 39229572 Free PMC article.
-
A human ex vivo skin model breaking boundaries.Sci Rep. 2024 Oct 14;14(1):24054. doi: 10.1038/s41598-024-75291-7. Sci Rep. 2024. PMID: 39402181 Free PMC article.
References
-
- Nakanishi T, et al., editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. Springer; 2016. - PubMed

