Effect of Antigen Structure in Subunit Vaccine Nanoparticles on Humoral Immune Responses
- PMID: 36848229
- PMCID: PMC10015428
- DOI: 10.1021/acsbiomaterials.2c01516
Effect of Antigen Structure in Subunit Vaccine Nanoparticles on Humoral Immune Responses
Abstract
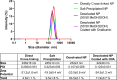
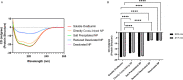
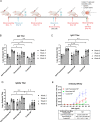
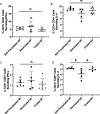
Subunit vaccines offer numerous attractive features, including good safety profiles and well-defined components with highly characterized properties because they do not contain whole pathogens. However, vaccine platforms based on one or few selected antigens are often poorly immunogenic. Several advances have been made in improving the effectiveness of subunit vaccines, including nanoparticle formulation and/or co-administration with adjuvants. Desolvation of antigens into nanoparticles is one approach that has been successful in eliciting protective immune responses. Despite this advance, damage to the antigen structure by desolvation can compromise the recognition of conformational antigens by B cells and the subsequent humoral response. Here, we used ovalbumin as a model antigen to demonstrate enhanced efficacy of subunit vaccines by preserving antigen structures in nanoparticles. An altered antigen structure due to desolvation was first validated by GROMACS and circular dichroism. Desolvant-free nanoparticles with a stable ovalbumin structure were successfully synthesized by directly cross-linking ovalbumin or using ammonium sulfate to form nanoclusters. Alternatively, desolvated OVA nanoparticles were coated with a layer of OVA after desolvation. Vaccination with salt-precipitated nanoparticles increased OVA-specific IgG titers 4.2- and 22-fold compared to the desolvated and coated nanoparticles, respectively. In addition, enhanced affinity maturation by both salt precipitated and coated nanoparticles was displayed in contrast to desolvated nanoparticles. These results demonstrate both that salt-precipitated antigen nanoparticles are a potential new vaccine platform with significantly improved humoral immunity and a functional value of preserving antigen structures in vaccine nanoparticle design.
Keywords: antigen structure; humoral immune response; nanoparticles; salt precipitation; subunit vaccine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
Cited by
-
Dual-Antigen Subunit Vaccine Nanoparticles for Scrub Typhus.Pathogens. 2023 Nov 25;12(12):1390. doi: 10.3390/pathogens12121390. Pathogens. 2023. PMID: 38133275 Free PMC article.
-
Chemical and biological conjugation strategies for the development of multivalent protein vaccine nanoparticles.Biopolymers. 2023 Aug;114(8):e23563. doi: 10.1002/bip.23563. Epub 2023 Jul 25. Biopolymers. 2023. PMID: 37490564 Free PMC article. Review.
-
Uptake Quantification of Antigen Carried by Nanoparticles and Its Impact on Carrier Adjuvanticity Evaluation.Vaccines (Basel). 2023 Dec 26;12(1):28. doi: 10.3390/vaccines12010028. Vaccines (Basel). 2023. PMID: 38250841 Free PMC article.
-
Development of Self-Assembled Protein Nanocage Spatially Functionalized with HA Stalk as a Broadly Cross-Reactive Influenza Vaccine Platform.ACS Nano. 2023 Dec 26;17(24):25045-25060. doi: 10.1021/acsnano.3c07669. Epub 2023 Dec 12. ACS Nano. 2023. PMID: 38084728 Free PMC article.
References
-
- Duan L.; Mukherjee E.. Janeway’s Immunobiology. Yale J Biol Med, 9th ed.; PMC, 2016; Vol. 89, pp 424–425
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

