Inhibitor of apoptosis proteins as therapeutic targets in bladder cancer
- PMID: 36845731
- PMCID: PMC9950391
- DOI: 10.3389/fonc.2023.1124600
Inhibitor of apoptosis proteins as therapeutic targets in bladder cancer
Abstract
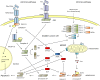
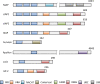
Evasion from apoptosis is a hallmark of cancer. Inhibitor of apoptosis proteins (IAPs) contribute to this hallmark by suppressing the induction of cell death. IAPs were found to be overexpressed in cancerous tissues and to contribute to therapeutic resistance. The present review focuses on the IAP members cIAP1, cIAP2, XIAP, Survivin and Livin and their importance as potential therapeutic targets in bladder cancer.
Keywords: Livin; Survivin; XIAP; apoptosis; bladder cancer; cIAP1; cIAP2; inhibitor of apoptosis proteins.
Copyright © 2023 Wolf.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Expression patterns of inhibitor of apoptosis proteins in malignant pleural mesothelioma.J Pathol. 2007 Mar;211(4):447-54. doi: 10.1002/path.2121. J Pathol. 2007. PMID: 17253596
-
The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways.Mol Biol Cell. 2008 Jul;19(7):2729-40. doi: 10.1091/mbc.e08-01-0107. Epub 2008 Apr 23. Mol Biol Cell. 2008. PMID: 18434593 Free PMC article.
-
Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies.Curr Mol Med. 2011 Nov;11(8):633-49. doi: 10.2174/156652411797536723. Curr Mol Med. 2011. PMID: 21902653 Review.
-
Targeting inhibitor of apoptosis proteins (IAPs) with IAP inhibitors sensitises malignant rhabdoid tumour cells to cisplatin.Cancer Treat Res Commun. 2022;32:100579. doi: 10.1016/j.ctarc.2022.100579. Epub 2022 May 18. Cancer Treat Res Commun. 2022. PMID: 35613525
-
Inhibitors of apoptotic proteins: new targets for anticancer therapy.Chem Biol Drug Des. 2013 Sep;82(3):243-51. doi: 10.1111/cbdd.12176. Chem Biol Drug Des. 2013. PMID: 23790005 Review.
Cited by
-
A Complex Pattern of Gene Expression in Tissue Affected by Viperid Snake Envenoming: The Emerging Role of Autophagy-Related Genes.Biomolecules. 2024 Feb 26;14(3):278. doi: 10.3390/biom14030278. Biomolecules. 2024. PMID: 38540699 Free PMC article.
-
Gold nanoparticles (AuNPs) decrease the viability of cervical cancer cells by inducing the BAX gene and activating antioxidant enzymes.Mol Biol Rep. 2024 Feb 8;51(1):287. doi: 10.1007/s11033-024-09253-7. Mol Biol Rep. 2024. PMID: 38329621
-
Docking and molecular dynamic simulations of Mithramycin-A and Tolfenamic acid against Sp1 and survivin.Process Biochem. 2024 Feb;137:207-216. doi: 10.1016/j.procbio.2023.12.014. Epub 2024 Jan 9. Process Biochem. 2024. PMID: 38912413
-
RNF135 Promotes Human Osteosarcoma Cell Growth and Inhibits Apoptosis by Upregulating the PI3K/AKT Pathway.Cancer Rep (Hoboken). 2024 Aug;7(8):e2159. doi: 10.1002/cnr2.2159. Cancer Rep (Hoboken). 2024. PMID: 39118262 Free PMC article.
-
Dendritic cells infected with recombinant adenoviral vector encoding mouse fibroblast activation protein-α and human livin α exert an antitumor effect against Lewis lung carcinoma in mice.Immun Inflamm Dis. 2023 Sep;11(9):e1011. doi: 10.1002/iid3.1011. Immun Inflamm Dis. 2023. PMID: 37773704 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

