Recent Advances in the Development of Nanodelivery Systems Targeting the TRAIL Death Receptor Pathway
- PMID: 36839837
- PMCID: PMC9961178
- DOI: 10.3390/pharmaceutics15020515
Recent Advances in the Development of Nanodelivery Systems Targeting the TRAIL Death Receptor Pathway
Abstract
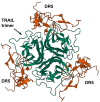
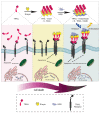
The TRAIL (TNF-related apoptosis-inducing ligand) apoptotic pathway is extensively exploited in the development of targeted antitumor therapy due to TRAIL specificity towards its cognate receptors, namely death receptors DR4 and DR5. Although therapies targeting the TRAIL pathway have encountered many obstacles in attempts at clinical implementation for cancer treatment, the unique features of the TRAIL signaling pathway continue to attract the attention of researchers. Special attention is paid to the design of novel nanoscaled delivery systems, primarily aimed at increasing the valency of the ligand for improved death receptor clustering that enhances apoptotic signaling. Optionally, complex nanoformulations can allow the encapsulation of several therapeutic molecules for a combined synergistic effect, for example, chemotherapeutic agents or photosensitizers. Scaffolds for the developed nanodelivery systems are fabricated by a wide range of conventional clinically approved materials and innovative ones, including metals, carbon, lipids, polymers, nanogels, protein nanocages, virus-based nanoparticles, dendrimers, DNA origami nanostructures, and their complex combinations. Most nanotherapeutics targeting the TRAIL pathway are aimed at tumor therapy and theranostics. However, given the wide spectrum of action of TRAIL due to its natural role in immune system homeostasis, other therapeutic areas are also involved, such as liver fibrosis, rheumatoid arthritis, Alzheimer's disease, and inflammatory diseases caused by bacterial infections. This review summarizes the recent innovative developments in the design of nanodelivery systems modified with TRAIL pathway-targeting ligands.
Keywords: DR5; TRAIL; death receptors; drug delivery; ligand-targeted drugs; nanoparticles; nanotherapeutics; receptor clustering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combination of TRAIL with bortezomib shifted apoptotic signaling from DR4 to DR5 death receptor by selective internalization and degradation of DR4.PLoS One. 2014 Oct 13;9(10):e109756. doi: 10.1371/journal.pone.0109756. eCollection 2014. PLoS One. 2014. PMID: 25310712 Free PMC article.
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
-
Death receptors and ligands in cervical carcinogenesis: an immunohistochemical study.Gynecol Oncol. 2005 Mar;96(3):705-13. doi: 10.1016/j.ygyno.2004.10.046. Gynecol Oncol. 2005. PMID: 15721415
-
Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5.Cancer Res. 2001 Mar 15;61(6):2704-12. Cancer Res. 2001. PMID: 11289151
-
Targeting Death Receptor 5 (DR5) for the imaging and treatment of primary bone and soft tissue tumors: an update of the literature.Front Mol Biosci. 2024 Sep 2;11:1384795. doi: 10.3389/fmolb.2024.1384795. eCollection 2024. Front Mol Biosci. 2024. PMID: 39286782 Free PMC article. Review.
Cited by
-
The Role of TRAIL Signaling in Cancer: Searching for New Therapeutic Strategies.Biology (Basel). 2024 Jul 15;13(7):521. doi: 10.3390/biology13070521. Biology (Basel). 2024. PMID: 39056714 Free PMC article. Review.
-
Focused ultrasound-induced cell apoptosis for the treatment of tumours.PeerJ. 2024 Aug 21;12:e17886. doi: 10.7717/peerj.17886. eCollection 2024. PeerJ. 2024. PMID: 39184389 Free PMC article. Review.
References
-
- Dufour F., Rattier T., Constantinescu A.A., Zischler L., Morlé A., Mabrouk H.B., Humblin E., Jacquemin G., Szegezdi E., Delacote F., et al. TRAIL Receptor Gene Editing Unveils TRAIL-R1 as a Master Player of Apoptosis Induced by TRAIL and ER Stress. Oncotarget. 2017;8:9974–9985. doi: 10.18632/oncotarget.14285. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

