The Impact of Antiviral Treatment of Hepatitis B Virus after Kidney Transplant and the Latest Insights
- PMID: 36839612
- PMCID: PMC9962423
- DOI: 10.3390/pathogens12020340
The Impact of Antiviral Treatment of Hepatitis B Virus after Kidney Transplant and the Latest Insights
Abstract
Background: The current frequency of hepatitis B virus infection in patients with advanced chronic kidney disease (CKD) (including patients on maintenance dialysis and kidney transplant recipients) is low but not negligible worldwide. HBV has a deleterious effect on survival after a kidney transplant; antiviral treatments improved the short-term outcomes of kidney transplant recipients, but their long-term impact remains uncertain.
Aim: The aim of this review is to assess the role of antiviral therapy for HBV in improving survival after a kidney transplant. The recent publication of large surveys has prompted us to update the available evidence on the impact of HBV on patient and graft survival after a kidney transplant.
Methods: We have conducted an extensive review of the medical literature, and various research engines have been used.
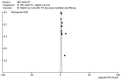
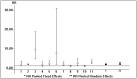
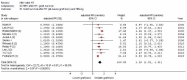
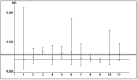
Results: We retrieved several studies (n = 11; n = 121,436 unique patients) and found an association between positive serologic HBsAg status and diminished patient and graft survival after a kidney transplant; the adjusted relative risk (aRR) of all-cause mortality and graft loss was 2.85 (95% CI, 2.36; 3.33, p < 0.0001) and 1.26 (95% CI, 1.02; 1.51, p < 0.0001), respectively. To our knowledge, at least six studies reported improved patient and graft survival after the adoption of antiviral therapies for HBV (this result was reported with both survival curves and multivariable regression). According to novel clinical guidelines, entecavir has been suggested as a 'first line' antiviral agent for the treatment of HBV after a kidney transplant.
Conclusions: The recent availability of safe and effective antiviral drugs for the treatment of HBV has meant that the survival curves of HBsAg-positive patients on antiviral therapy and HBsAg-negative patients after a kidney transplant can be comparable. Antiviral therapy should be systematically proposed to HBV-positive kidney transplant recipients and candidates to avoid the deleterious hepatic and extra-hepatic effects of chronic HBV replication.
Keywords: hepatitis B virus; kidney transplantation; nucleos(t)ide analogues; survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Control of replication of hepatitis B and C virus improves patient and graft survival in kidney transplantation.J Hepatol. 2019 May;70(5):831-838. doi: 10.1016/j.jhep.2018.12.036. Epub 2019 Mar 14. J Hepatol. 2019. PMID: 30879789
-
Hepatitis B virus among hematopoietic stem cell transplant recipients: Antiviral impact in seroconversion, engraftment, and mortality in a Latin American center.Transpl Infect Dis. 2020 Apr;22(2):e13243. doi: 10.1111/tid.13243. Epub 2020 Jan 13. Transpl Infect Dis. 2020. PMID: 31901206
-
Successful withdrawal of antiviral treatment in kidney transplant recipients with chronic hepatitis B viral infection.Transpl Infect Dis. 2014 Apr;16(2):295-303. doi: 10.1111/tid.12202. Epub 2014 Mar 17. Transpl Infect Dis. 2014. PMID: 24628837
-
Treatment of chronic hepatitis B virus infection - Dutch national guidelines.Neth J Med. 2008 Jul-Aug;66(7):292-306. Neth J Med. 2008. PMID: 18663260 Review.
-
[Evaluation of viral hepatitis in solid organ transplantation].Acta Med Croatica. 2014 Apr;68(2):151-9. Acta Med Croatica. 2014. PMID: 26012153 Review. Croatian.
References
-
- Lampertico P., Agarwal K., Berg T., Buti M., Janssen H.L., Papatheodoridis G., Zoulim F., Tacke F. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. European Association for the Study of the Liver. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

