MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism
- PMID: 36835623
- PMCID: PMC9966807
- DOI: 10.3390/ijms24044218
MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism
Abstract
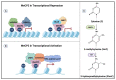
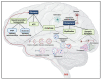
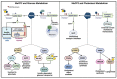
DNA methylation, one of the most well-studied epigenetic modifications, is involved in a wide spectrum of biological processes. Epigenetic mechanisms control cellular morphology and function. Such regulatory mechanisms involve histone modifications, chromatin remodeling, DNA methylation, non-coding regulatory RNA molecules, and RNA modifications. One of the most well-studied epigenetic modifications is DNA methylation that plays key roles in development, health, and disease. Our brain is probably the most complex part of our body, with a high level of DNA methylation. A key protein that binds to different types of methylated DNA in the brain is the methyl-CpG binding protein 2 (MeCP2). MeCP2 acts in a dose-dependent manner and its abnormally high or low expression level, deregulation, and/or genetic mutations lead to neurodevelopmental disorders and aberrant brain function. Recently, some of MeCP2-associated neurodevelopmental disorders have emerged as neurometabolic disorders, suggesting a role for MeCP2 in brain metabolism. Of note, MECP2 loss-of-function mutation in Rett Syndrome is reported to cause impairment of glucose and cholesterol metabolism in human patients and/or mouse models of disease. The purpose of this review is to outline the metabolic abnormalities in MeCP2-associated neurodevelopmental disorders that currently have no available cure. We aim to provide an updated overview into the role of metabolic defects associated with MeCP2-mediated cellular function for consideration of future therapeutic strategies.
Keywords: AMPK; BDNF; DNA methylation; MeCP2 isoforms; Rett Syndrome; autophagy; brain development; brain metabolism; cholesterol; epigenetics; glucose; mTOR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MeCP2 binds to non-CG methylated DNA as neurons mature, influencing transcription and the timing of onset for Rett syndrome.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5509-14. doi: 10.1073/pnas.1505909112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870282 Free PMC article.
-
Rett syndrome and MeCP2.Neuromolecular Med. 2014 Jun;16(2):231-64. doi: 10.1007/s12017-014-8295-9. Epub 2014 Mar 11. Neuromolecular Med. 2014. PMID: 24615633 Free PMC article. Review.
-
MeCP2, A Modulator of Neuronal Chromatin Organization Involved in Rett Syndrome.Adv Exp Med Biol. 2017;978:3-21. doi: 10.1007/978-3-319-53889-1_1. Adv Exp Med Biol. 2017. PMID: 28523538 Review.
-
MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression.Hum Mol Genet. 2005 Apr 15;14(8):1049-58. doi: 10.1093/hmg/ddi097. Epub 2005 Mar 9. Hum Mol Genet. 2005. PMID: 15757975
-
Transcriptomic and Epigenomic Landscape in Rett Syndrome.Biomolecules. 2021 Jun 30;11(7):967. doi: 10.3390/biom11070967. Biomolecules. 2021. PMID: 34209228 Free PMC article. Review.
Cited by
-
Drug repurposing in Rett and Rett-like syndromes: a promising yet underrated opportunity?Front Med (Lausanne). 2024 Jul 29;11:1425038. doi: 10.3389/fmed.2024.1425038. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39135718 Free PMC article.
-
Potential for New Therapeutic Approaches by Targeting Lactate and pH Mediated Epigenetic Dysregulation in Major Mental Diseases.Biomedicines. 2024 Feb 18;12(2):457. doi: 10.3390/biomedicines12020457. Biomedicines. 2024. PMID: 38398057 Free PMC article. Review.
-
The Epigenetic Reader Methyl-CpG-Binding Protein 2 (MeCP2) Is an Emerging Oncogene in Cancer Biology.Cancers (Basel). 2023 May 9;15(10):2683. doi: 10.3390/cancers15102683. Cancers (Basel). 2023. PMID: 37345019 Free PMC article. Review.
-
Neurotrophins and Their Receptors: BDNF's Role in GABAergic Neurodevelopment and Disease.Int J Mol Sci. 2024 Jul 30;25(15):8312. doi: 10.3390/ijms25158312. Int J Mol Sci. 2024. PMID: 39125882 Free PMC article. Review.
-
Central Causation of Autism/ASDs via Excessive [Ca2+]i Impacting Six Mechanisms Controlling Synaptogenesis during the Perinatal Period: The Role of Electromagnetic Fields and Chemicals and the NO/ONOO(-) Cycle, as Well as Specific Mutations.Brain Sci. 2024 Apr 30;14(5):454. doi: 10.3390/brainsci14050454. Brain Sci. 2024. PMID: 38790433 Free PMC article. Review.
References
-
- Liyanage V.R.B., Zachariah R.M., Delcuve G.P., Davie J.R., Rastegar M. Chromatin Structure and Epigenetics. In: Urbano K.V., editor. Advances in Genetics Research. Volume 13. Nova Science Publishers; Hauppauge, NY, USA: 2015. pp. 57–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

