High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke
- PMID: 36831312
- PMCID: PMC9954836
- DOI: 10.3390/cells12040645
High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke
Abstract
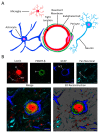
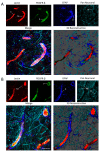
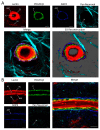
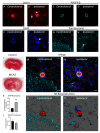
The neurovascular unit (NVU) is an anatomical group of cells that establishes the blood-brain barrier (BBB) and coordinates cerebral blood flow in association with neuronal function. In cerebral gray matter, cellular constituents of the NVU include endothelial cells and associated pericytes, astrocytes, neurons, and microglia. Dysfunction of the NVU is a common feature of diseases that affect the CNS, such as ischemic stroke. High-level evaluation of these NVU changes requires the use of imaging modalities that can enable the visualization of various cell types under disease conditions. In this study, we applied our confocal microscopy strategy using commercially available labeling reagents to, for the first time, simultaneously investigate associations between endothelial cells, the vascular basal lamina, pericytes, microglia, astrocytes and/or astrocyte end-feet, and neurites in both healthy and ischemic brain tissue. This allowed us to demonstrate ischemia-induced astrocyte activation, neurite loss, and microglial migration toward blood vessels in a single confocal image. Furthermore, our labeling cocktail enabled a precise quantification of changes in neurites and astrocyte reactivity, thereby showing the relationship between different NVU cellular constituents in healthy and diseased brain tissue. The application of our imaging approach for the simultaneous visualization of multiple NVU cell types provides an enhanced understanding of NVU function and pathology, a state-of-the-art advancement that will facilitate the development of more effective treatment strategies for diseases of the CNS that exhibit neurovascular dysfunction, such as ischemic stroke.
Keywords: astrocytes; endothelial cells; ischemic stroke; microglia; neurons; neurovascular unit; pericytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke.Acta Neuropathol. 2022 Aug;144(2):305-337. doi: 10.1007/s00401-022-02452-1. Epub 2022 Jun 25. Acta Neuropathol. 2022. PMID: 35752654 Free PMC article.
-
Crosstalk Among Glial Cells in the Blood-Brain Barrier Injury After Ischemic Stroke.Mol Neurobiol. 2024 Sep;61(9):6161-6174. doi: 10.1007/s12035-024-03939-6. Epub 2024 Jan 27. Mol Neurobiol. 2024. PMID: 38279077 Review.
-
Modeling ischemic stroke in a triculture neurovascular unit on-a-chip.Fluids Barriers CNS. 2021 Dec 14;18(1):59. doi: 10.1186/s12987-021-00294-9. Fluids Barriers CNS. 2021. PMID: 34906183 Free PMC article.
-
Targetability of the neurovascular unit in inflammatory diseases of the central nervous system.Immunol Rev. 2022 Oct;311(1):39-49. doi: 10.1111/imr.13121. Epub 2022 Jul 31. Immunol Rev. 2022. PMID: 35909222 Free PMC article. Review.
-
Reconstituting neurovascular unit with primary neural stem cells and brain microvascular endothelial cells in three-dimensional matrix.Brain Pathol. 2021 Sep;31(5):e12940. doi: 10.1111/bpa.12940. Epub 2021 Feb 12. Brain Pathol. 2021. PMID: 33576166 Free PMC article.
Cited by
-
Oatp (Organic Anion Transporting Polypeptide)-Mediated Transport: A Mechanism for Atorvastatin Neuroprotection in Stroke.Stroke. 2023 Nov;54(11):2875-2885. doi: 10.1161/STROKEAHA.123.043649. Epub 2023 Sep 26. Stroke. 2023. PMID: 37750296 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

