Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial
- PMID: 36831183
- PMCID: PMC9954596
- DOI: 10.3390/cells12040516
Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial
Abstract
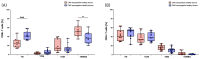
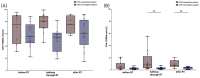
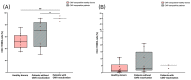
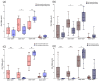
Radiotherapy (RT) of the brain is a common treatment for patients with high-grade gliomas and brain metastases. It has previously been shown that reactivation of cytomegalovirus (CMV) frequently occurs during RT of the brain. This causes neurological decline, demands antiviral treatment, and is associated with a worse prognosis. CMV-specific T cells are characterized by a differentiated effector memory phenotype and CD45RA+ CCR7- effector memory T (TEMRA) cells were shown to be enriched in CMV seropositive individuals. In this study, we investigated the distribution of TEMRA cells and their subsets in the peripheral blood of healthy donors and, for the first time, prospectively within the scope of the prospective Glio-CMV-01 clinical trial of patients with high-grade glioma and brain metastases during radiation therapy as a potential predictive marker. First, we developed a multicolor flow cytometry-based assay to monitor the frequency and distribution of TEMRA cells in a longitudinal manner. The CMV serostatus and age were considered as influencing factors. We revealed that patients who had a reactivation of CMV have significantly higher amounts of CD8+ TEMRA cells. Further, the distribution of the subsets of TEMRA cells based on the expression of CD27, CD28, and CD57 is highly dependent on the CMV serostatus. We conclude that the percentage of CD8+ TEMRA cells out of all CD8+ T cells has the potential to serve as a biomarker for predicting the risk of CMV reactivation during RT of the brain. Furthermore, this study highlights the importance of taking the CMV serostatus into account when analyzing TEMRA cells and their subsets.
Keywords: TEMRA cells; brain metastases; cytomegalovirus (CMV); glioblastoma; infection; radiotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cytotoxic CD8+ Temra cells show loss of chromatin accessibility at genes associated with T cell activation.Front Immunol. 2024 Feb 2;15:1285798. doi: 10.3389/fimmu.2024.1285798. eCollection 2024. Front Immunol. 2024. PMID: 38370415 Free PMC article.
-
Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans.J Gen Virol. 2011 Dec;92(Pt 12):2746-2756. doi: 10.1099/vir.0.036004-0. Epub 2011 Aug 3. J Gen Virol. 2011. PMID: 21813708
-
Rapid Acquisition of Cytomegalovirus-Specific T Cells with a Differentiated Phenotype, in Nonviremic Hematopoietic Stem Transplant Recipients Vaccinated with CMVPepVax.Biol Blood Marrow Transplant. 2019 Apr;25(4):771-784. doi: 10.1016/j.bbmt.2018.12.070. Epub 2018 Dec 16. Biol Blood Marrow Transplant. 2019. PMID: 30562587 Free PMC article.
-
Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: A systematic review.Exp Gerontol. 2016 May;77:87-95. doi: 10.1016/j.exger.2016.02.005. Epub 2016 Feb 13. Exp Gerontol. 2016. PMID: 26883338 Review.
-
Terminally differentiated effector memory T cells in kidney transplant recipients: New crossroads.Am J Transplant. 2024 Oct 9:S1600-6135(24)00629-4. doi: 10.1016/j.ajt.2024.10.001. Online ahead of print. Am J Transplant. 2024. PMID: 39389314 Review.
Cited by
-
Clinical implications of cytomegalovirus in glioblastoma progression and therapy.NPJ Precis Oncol. 2024 Sep 29;8(1):213. doi: 10.1038/s41698-024-00709-4. NPJ Precis Oncol. 2024. PMID: 39343770 Free PMC article. Review.
References
-
- Sodeik B., Messerle M., Schulz T.F. Herpesviren. In: Suerbaum S., Burchard G.-D., Kaufmann S.H.E., Schulz T.F., editors. Medizinische Mikrobiologie und Infektiologie. Springer; Berlin/Heidelberg, Germany: 2020. pp. 723–747.
-
- Goerig N.L., Frey B., Korn K., Fleckenstein B., Uberla K., Schmidt M.A., Dorfler A., Engelhorn T., Eyupoglu I., Ruhle P.F., et al. Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro-Oncology. 2016;18:1664–1672. doi: 10.1093/neuonc/now120. - DOI - PMC - PubMed
-
- Goerig N.L., Frey B., Korn K., Fleckenstein B., Uberla K., Schmidt M.A., Dorfler A., Engelhorn T., Eyupoglu I., Ruhle P.F., et al. Early Mortality of Brain Cancer Patients and its Connection to Cytomegalovirus Reactivation During Radiochemotherapy. Clin. Cancer Res. 2020;26:3259–3270. doi: 10.1158/1078-0432.CCR-19-3195. - DOI - PubMed
-
- Bodensohn R., Kaempfel A.L., Fleischmann D.F., Hadi I., Hofmaier J., Garny S., Reiner M., Forbrig R., Corradini S., Thon N., et al. Simultaneous stereotactic radiosurgery of multiple brain metastases using single-isocenter dynamic conformal arc therapy: A prospective monocentric registry trial. Strahlenther. Onkol. 2021;197:601–613. doi: 10.1007/s00066-021-01773-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

