Modulating Morphological and Redox/Glycative Alterations in the PCOS Uterus: Effects of Carnitines in PCOS Mice
- PMID: 36830911
- PMCID: PMC9953026
- DOI: 10.3390/biomedicines11020374
Modulating Morphological and Redox/Glycative Alterations in the PCOS Uterus: Effects of Carnitines in PCOS Mice
Abstract
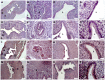
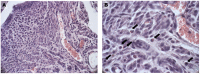
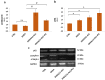
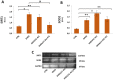
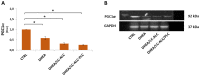
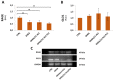
(1) Background: Polycystic ovarian syndrome (PCOS) is a common and multifactorial disease affecting reproductive-age women. Although PCOS ovarian and metabolic features have received extensive research, uterine dysfunction has been poorly investigated. This research aims to investigate morphological and molecular alterations in the PCOS uterus and search for modulating effects of different carnitine formulations. (2) Methods: CD1 mice were administered or not with dehydroepiandrosterone (DHEA, 6 mg/100 g body weight) for 20 days, alone or with 0.40 mg L-carnitine (LC) and 0.20 mg acetyl-L-carnitine (ALC) in the presence or absence of 0.08 mg propionyl-L-carnitine (PLC). Uterine horns from the four groups were subjected to histology, immunohistochemistry and immunoblotting analyses to evaluate their morphology, collagen deposition, autophagy and steroidogenesis. Oxidative-/methylglyoxal (MG)-dependent damage was investigated along with the effects on the mitochondria, SIRT1, SOD2, RAGE and GLO1 proteins. (3) Results: The PCOS uterus suffers from tissue and oxidative alterations associated with MG-AGE accumulation. LC-ALC administration alleviated PCOS uterine tissue alterations and molecular damage. The presence of PLC prevented fibrosis and maintained mitochondria content. (4) Conclusions: The present results provide evidence for oxidative and glycative damage as the main factors contributing to PCOS uterine alterations and include the uterus in the spectrum of action of carnitines on the PCOS phenotype.
Keywords: DHEA; PCOS; SIRT1; carnitines; glycative stress; methylglyoxal; mitochondria; mouse; oxidative stress; uterus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Acyl-Carnitines Exert Positive Effects on Mitochondrial Activity under Oxidative Stress in Mouse Oocytes: A Potential Mechanism Underlying Carnitine Efficacy on PCOS.Biomedicines. 2023 Sep 6;11(9):2474. doi: 10.3390/biomedicines11092474. Biomedicines. 2023. PMID: 37760915 Free PMC article.
-
Regulatory Functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: Focus on Antioxidant/Antiglycative Molecular Pathways in the Ovarian Microenvironment.Antioxidants (Basel). 2020 Sep 15;9(9):867. doi: 10.3390/antiox9090867. Antioxidants (Basel). 2020. PMID: 32942589 Free PMC article.
-
Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice.Cells. 2020 Jan 14;9(1):209. doi: 10.3390/cells9010209. Cells. 2020. PMID: 31947651 Free PMC article.
-
Carnitines as Mitochondrial Modulators of Oocyte and Embryo Bioenergetics.Antioxidants (Basel). 2022 Apr 8;11(4):745. doi: 10.3390/antiox11040745. Antioxidants (Basel). 2022. PMID: 35453430 Free PMC article. Review.
-
Uterine Function: From Normal to Polycystic Ovarian Syndrome Alterations.Curr Med Chem. 2018;25(15):1792-1804. doi: 10.2174/0929867325666171205144119. Curr Med Chem. 2018. PMID: 29210631 Review.
Cited by
-
Acyl-Carnitines Exert Positive Effects on Mitochondrial Activity under Oxidative Stress in Mouse Oocytes: A Potential Mechanism Underlying Carnitine Efficacy on PCOS.Biomedicines. 2023 Sep 6;11(9):2474. doi: 10.3390/biomedicines11092474. Biomedicines. 2023. PMID: 37760915 Free PMC article.
-
Mitochondrial Dysfunction in PCOS: Insights into Reproductive Organ Pathophysiology.Int J Mol Sci. 2023 Aug 23;24(17):13123. doi: 10.3390/ijms241713123. Int J Mol Sci. 2023. PMID: 37685928 Free PMC article. Review.
References
-
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Futterweit W., Janssen O.E., Legro R.S., Norman R.J., Taylor A.E., et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil. Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

