Mead acid inhibits retinol-induced irritant contact dermatitis via peroxisome proliferator-activated receptor alpha
- PMID: 36825199
- PMCID: PMC9941550
- DOI: 10.3389/fmolb.2023.1097955
Mead acid inhibits retinol-induced irritant contact dermatitis via peroxisome proliferator-activated receptor alpha
Abstract
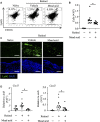
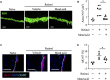
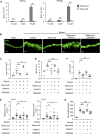
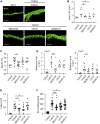
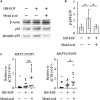
Retinol is widely used in topical skincare products to ameliorate skin aging and treat acne and wrinkles; however, retinol and its derivatives occasionally have adverse side effects, including the induction of irritant contact dermatitis. Previously, we reported that mead acid (5,8,11-eicosatrienoic acid), an oleic acid metabolite, ameliorated skin inflammation in dinitrofluorobenzene-induced allergic contact hypersensitivity by inhibiting neutrophil infiltration and leukotriene B4 production by neutrophils. Here, we showed that mead acid also suppresses retinol-induced irritant contact dermatitis. In a murine model, we revealed that mead acid inhibited keratinocyte abnormalities such as keratinocyte hyperproliferation. Consistently, mead acid inhibited p38 MAPK (mitogen-activated protein kinase) phosphorylation, which is an essential signaling pathway in the keratinocyte hyperplasia induced by retinol. These inhibitory effects of mead acid were associated with the prevention of both keratinocyte hyperproliferation and the gene expression of neutrophil chemoattractants, including Cxcl1 and Cxcl2, and they were mediated by a PPAR (peroxisome proliferator-activated receptor)-α pathway. Our findings identified the anti-inflammatory effects of mead acid, the use of which can be expected to minimize the risk of adverse side effects associated with topical retinoid application.
Keywords: hyperproliferation; inflammation; irritant contact dermatitis (ICD); keratinocyte; lipid metabolite; mead acid; oleic acid; retinol.
Copyright © 2023 Saika, Tiwari, Nagatake, Node, Hosomi, Honda, Kabashima and Kunisawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Topical peroxisome proliferator activated receptor-alpha activators reduce inflammation in irritant and allergic contact dermatitis models.J Invest Dermatol. 2002 Jan;118(1):94-101. doi: 10.1046/j.0022-202x.2001.01626.x. J Invest Dermatol. 2002. PMID: 11851881
-
Dietary coconut oil ameliorates skin contact hypersensitivity through mead acid production in mice.Allergy. 2019 Aug;74(8):1522-1532. doi: 10.1111/all.13762. Epub 2019 Apr 10. Allergy. 2019. PMID: 30843234
-
ω3 fatty acid metabolite, 12-hydroxyeicosapentaenoic acid, alleviates contact hypersensitivity by downregulation of CXCL1 and CXCL2 gene expression in keratinocytes via retinoid X receptor α.FASEB J. 2021 Apr;35(4):e21354. doi: 10.1096/fj.202001687R. FASEB J. 2021. PMID: 33749892
-
Contact dermatitis to topical acne drugs: a review of the literature.Dermatol Ther. 2015 Sep-Oct;28(5):323-9. doi: 10.1111/dth.12282. Epub 2015 Aug 24. Dermatol Ther. 2015. PMID: 26302055 Review.
-
Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology.J Lipid Res. 2008 Mar;49(3):499-509. doi: 10.1194/jlr.R800001-JLR200. Epub 2008 Jan 8. J Lipid Res. 2008. PMID: 18182682 Review.
Cited by
-
Long Non-Coding RNAs, Nuclear Receptors and Their Cross-Talks in Cancer-Implications and Perspectives.Cancers (Basel). 2024 Aug 22;16(16):2920. doi: 10.3390/cancers16162920. Cancers (Basel). 2024. PMID: 39199690 Free PMC article. Review.
-
The physiological and pathological properties of Mead acid, an endogenous multifunctional n-9 polyunsaturated fatty acid.Lipids Health Dis. 2023 Oct 14;22(1):172. doi: 10.1186/s12944-023-01937-6. Lipids Health Dis. 2023. PMID: 37838679 Free PMC article. Review.
References
-
- Cattani F., Gallese A., Mosca M., Buanne P., Biordi L., Francavilla S., et al. (2006). The role of CXCR2 activity in the contact hypersensitivity response in mice. Eur. Cytokine Netw. 17, 42–48. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

