Bioinformatics analysis and consistency verification of a novel tuberculosis vaccine candidate HP13138PB
- PMID: 36825009
- PMCID: PMC9942524
- DOI: 10.3389/fimmu.2023.1102578
Bioinformatics analysis and consistency verification of a novel tuberculosis vaccine candidate HP13138PB
Erratum in
-
Corrigendum: Bioinformatics analysis and consistency verification of a novel tuberculosis vaccine candidate HP13138PB.Front Immunol. 2023 Feb 8;14:1154693. doi: 10.3389/fimmu.2023.1154693. eCollection 2023. Front Immunol. 2023. PMID: 36845146 Free PMC article.
Abstract
Background: With the increasing incidence of tuberculosis (TB) and the shortcomings of existing TB vaccines to prevent TB in adults, new TB vaccines need to be developed to address the complex TB epidemic.
Method: The dominant epitopes were screened from antigens to construct a novel epitope vaccine termed HP13138PB. The immune properties, structure, and function of HP13138PB were predicted and analyzed with bioinformatics and immunoinformatics. Then, the immune responses induced by the HP13138PB were confirmed by enzyme-linked immunospot assay (ELISPOT) and Th1/Th2/Th17 multi-cytokine detection kit.
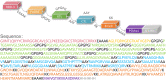
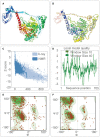
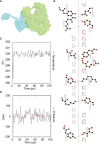
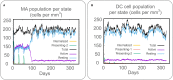
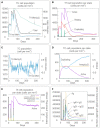
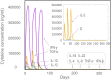
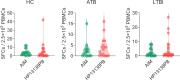
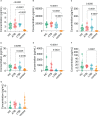
Result: The HP13138PB vaccine consisted of 13 helper T lymphocytes (HTL) epitopes, 13 cytotoxic T lymphocytes (CTL) epitopes, and 8 B-cell epitopes. It was found that the antigenicity, immunogenicity, and solubility index of the HP13138PB vaccine were 0.87, 2.79, and 0.55, respectively. The secondary structure prediction indicated that the HP13138PB vaccine had 31% of α-helix, 11% of β-strand, and 56% of coil. The tertiary structure analysis suggested that the Z-score and the Favored region of the HP13138PB vaccine were -4.47 88.22%, respectively. Furthermore, the binding energies of the HP13138PB to toll-like receptor 2 (TLR2) was -1224.7 kcal/mol. The immunoinformatics and real-world experiments showed that the HP13138PB vaccine could induce an innate and adaptive immune response characterized by significantly higher levels of cytokines such as interferon-gamma (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), and IL-10.
Conclusion: The HP13138PB is a potential vaccine candidate to prevent TB, and this study preliminarily evaluated the ability of the HP13138PB to generate an immune response, providing a precursor target for developing TB vaccines.
Keywords: bioinformatics; epitope vaccines; immune responses; immunoinformatics; tuberculosis.
Copyright © 2023 Cheng, Jiang, Wang, Wang, Xue, Wang and Gong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A comprehensive approach to developing a multi-epitope vaccine against Mycobacterium tuberculosis: from in silico design to in vitro immunization evaluation.Front Immunol. 2023 Nov 2;14:1280299. doi: 10.3389/fimmu.2023.1280299. eCollection 2023. Front Immunol. 2023. PMID: 38022558 Free PMC article.
-
PP19128R, a Multiepitope Vaccine Designed to Prevent Latent Tuberculosis Infection, Induced Immune Responses In Silico and In Vitro Assays.Vaccines (Basel). 2023 Apr 17;11(4):856. doi: 10.3390/vaccines11040856. Vaccines (Basel). 2023. PMID: 37112768 Free PMC article.
-
Evaluation of the consistence between the results of immunoinformatics predictions and real-world animal experiments of a new tuberculosis vaccine MP3RT.Front Cell Infect Microbiol. 2022 Nov 2;12:1047306. doi: 10.3389/fcimb.2022.1047306. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36405961 Free PMC article.
-
Development a multi-epitope driven subunit vaccine for immune response reinforcement against Serogroup B of Neisseria meningitidis using comprehensive immunoinformatics approaches.Infect Genet Evol. 2019 Nov;75:103992. doi: 10.1016/j.meegid.2019.103992. Epub 2019 Aug 5. Infect Genet Evol. 2019. PMID: 31394292
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Development of multi-epitope mRNA vaccine against Clostridioides difficile using reverse vaccinology and immunoinformatics approaches.Synth Syst Biotechnol. 2024 May 18;9(4):667-683. doi: 10.1016/j.synbio.2024.05.008. eCollection 2024 Dec. Synth Syst Biotechnol. 2024. PMID: 38817826 Free PMC article.
-
Next-Generation TB Vaccines: Progress, Challenges, and Prospects.Vaccines (Basel). 2023 Jul 31;11(8):1304. doi: 10.3390/vaccines11081304. Vaccines (Basel). 2023. PMID: 37631874 Free PMC article. Review.
-
Impact of diabetes mellitus on tuberculosis prevention, diagnosis, and treatment from an immunologic perspective.Exploration (Beijing). 2024 Mar 5;4(5):20230138. doi: 10.1002/EXP.20230138. eCollection 2024 Oct. Exploration (Beijing). 2024. PMID: 39439490 Free PMC article. Review.
-
A comprehensive approach to developing a multi-epitope vaccine against Mycobacterium tuberculosis: from in silico design to in vitro immunization evaluation.Front Immunol. 2023 Nov 2;14:1280299. doi: 10.3389/fimmu.2023.1280299. eCollection 2023. Front Immunol. 2023. PMID: 38022558 Free PMC article.
-
Excluding Participants With Mycobacteria Infections From Clinical Trials: A Critical Consideration in Evaluating the Efficacy of BCG Against COVID-19.J Korean Med Sci. 2023 Oct 30;38(42):e343. doi: 10.3346/jkms.2023.38.e343. J Korean Med Sci. 2023. PMID: 37904656 Free PMC article. Review.
References
-
- WHO . Global tuberculosis report 2022. Geneva: World Health Organization: Genevapp: World Health Organization; (2022).
-
- Huang F, Zhao Y. Global control of tuberculosis: Current status and future prospects. In: Zoonoses (2021) 2:21. doi: 10.15212/ZOONOSES-2021-0021 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

