Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma
- PMID: 36822672
- PMCID: PMC9950980
- DOI: 10.1136/jitc-2022-005968
Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma
Abstract
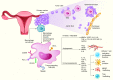
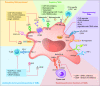
Epithelial ovarian cancer (EOC) is among the top five causes of cancer-related death in women, largely reflecting early, prediagnosis dissemination of malignant cells to the peritoneum. Despite improvements in medical therapies, particularly with the implementation of novel drugs targeting homologous recombination deficiency, the survival rates of patients with EOC remain low. Unlike other neoplasms, EOC remains relatively insensitive to immune checkpoint inhibitors, which is correlated with a tumor microenvironment (TME) characterized by poor infiltration by immune cells and active immunosuppression dominated by immune components with tumor-promoting properties, especially tumor-associated macrophages (TAMs). In recent years, TAMs have attracted interest as potential therapeutic targets by seeking to reverse the immunosuppression in the TME and enhance the clinical efficacy of immunotherapy. Here, we review the key biological features of TAMs that affect tumor progression and their relevance as potential targets for treating EOC. We especially focus on the therapies that might modulate the recruitment, polarization, survival, and functional properties of TAMs in the TME of EOC that can be harnessed to develop superior combinatorial regimens with immunotherapy for the clinical care of patients with EOC.
Keywords: Immunomodulation; Immunotherapy; Macrophages; Tumor Biomarkers; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RS is minority shareholder of Sotio Biotech a.s. JF and IT are employees of Sotio Biotech.
Figures
Similar articles
-
Immunological configuration of ovarian carcinoma: features and impact on disease outcome.J Immunother Cancer. 2021 Oct;9(10):e002873. doi: 10.1136/jitc-2021-002873. J Immunother Cancer. 2021. PMID: 34645669 Free PMC article. Review.
-
Extracellular vesicle-packaged miR-181c-5p from epithelial ovarian cancer cells promotes M2 polarization of tumor-associated macrophages via the KAT2B/HOXA10 axis.J Gene Med. 2022 Oct;24(10):e3446. doi: 10.1002/jgm.3446. Epub 2022 Sep 19. J Gene Med. 2022. PMID: 36027869
-
Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer.Front Immunol. 2022 Aug 10;13:956224. doi: 10.3389/fimmu.2022.956224. eCollection 2022. Front Immunol. 2022. PMID: 36032075 Free PMC article.
-
Tumor-associated macrophages and the tumor immune microenvironment of primary and recurrent epithelial ovarian cancer.Hum Pathol. 2018 Apr;74:135-147. doi: 10.1016/j.humpath.2017.12.010. Epub 2017 Dec 27. Hum Pathol. 2018. PMID: 29288043
-
Therapeutic implications of the tumor microenvironment in ovarian cancer patients receiving PD-1/PD-L1 therapy.Front Immunol. 2022 Oct 20;13:1036298. doi: 10.3389/fimmu.2022.1036298. eCollection 2022. Front Immunol. 2022. PMID: 36341388 Free PMC article. Review.
Cited by
-
Functional analysis and validation of oncodrive gene AP3S1 in ovarian cancer through filtering of mutation data from whole-exome sequencing.Eur J Med Res. 2024 Apr 12;29(1):231. doi: 10.1186/s40001-024-01814-7. Eur J Med Res. 2024. PMID: 38609993 Free PMC article.
-
Role of Tumor-Associated Macrophages in Cervical Cancer: Integrating Classical Perspectives with Recent Technological Advances.Life (Basel). 2024 Mar 27;14(4):443. doi: 10.3390/life14040443. Life (Basel). 2024. PMID: 38672714 Free PMC article. Review.
-
CCT2 prevented β-catenin proteasomal degradation to sustain cancer stem cell traits and promote tumor progression in epithelial ovarian cancer.Mol Biol Rep. 2024 Jan 2;51(1):54. doi: 10.1007/s11033-023-09047-3. Mol Biol Rep. 2024. PMID: 38165547
-
Chemotherapy induces myeloid-driven spatial T-cell exhaustion in ovarian cancer.bioRxiv [Preprint]. 2024 Mar 20:2024.03.19.585657. doi: 10.1101/2024.03.19.585657. bioRxiv. 2024. Update in: Cancer Cell. 2024 Dec 9;42(12):2045-2063.e10. doi: 10.1016/j.ccell.2024.11.005 PMID: 38562799 Free PMC article. Updated. Preprint.
-
Hypoxia-driven M2-polarized macrophages facilitate the epithelial-mesenchymal transition of glioblastoma via extracellular vesicles.Theranostics. 2024 Oct 7;14(16):6392-6408. doi: 10.7150/thno.95766. eCollection 2024. Theranostics. 2024. PMID: 39431006 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
