Predicting structural features of selected flavonoids responsible for neuroprotection in a Drosophila model of Parkinson's disease
- PMID: 36822376
- PMCID: PMC11080622
- DOI: 10.1016/j.neuro.2023.02.008
Predicting structural features of selected flavonoids responsible for neuroprotection in a Drosophila model of Parkinson's disease
Abstract
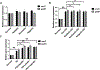
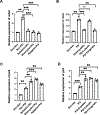
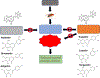
Nature-derived bioactive compounds have emerged as promising candidates for the prevention and treatment of diverse chronic illnesses, including neurodegenerative diseases. However, the exact molecular mechanisms underlying their neuroprotective effects remain unclear. Most studies focus solely on the antioxidant activities of natural products which translate to poor outcome in clinical trials. Current therapies against neurodegeneration only provide symptomatic relief, thereby underscoring the need for novel strategies to combat disease onset and progression. We have employed an environmental toxin-induced Drosophila Parkinson's disease (PD) model as an inexpensive in vivo screening platform to explore the neuroprotective potential of selected dietary flavonoids. We have identified a specific group of flavonoids known as flavones displaying protection against paraquat (PQ)-induced neurodegenerative phenotypes involving reduced survival, mobility defects, and enhanced oxidative stress. Interestingly, the other groups of investigated flavonoids, namely, the flavonones and flavonols failed to provide protection indicating a requirement of specific structural features that confer protection against PQ-mediated neurotoxicity in Drosophila. Based on our screen, the neuroprotective flavones lack a functional group substitution at the C3 and contain α,β-unsaturated carbonyl group. Furthermore, flavones-mediated neuroprotection is not solely dependent on antioxidant properties through nuclear factor erythroid 2-related factor 2 (Nrf2) but also requires regulation of the immune deficiency (IMD) pathway involving NFκB and the negative regulator poor Imd response upon knock-in (Pirk). Our data have identified specific structural features of selected flavonoids that provide neuroprotection against environmental toxin-induced PD pathogenesis that can be explored for novel therapeutic interventions.
Keywords: Drosophila; Flavonoid; Neuroinflammation; Neuroprotection; Parkinson’s disease.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Declaration of competing interest None.
Figures



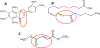
Similar articles
-
GardeninA confers neuroprotection against environmental toxin in a Drosophila model of Parkinson's disease.Commun Biol. 2021 Feb 5;4(1):162. doi: 10.1038/s42003-021-01685-2. Commun Biol. 2021. PMID: 33547411 Free PMC article.
-
Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer's Disease and Parkinson's Disease.Int J Mol Sci. 2019 Jul 10;20(14):3380. doi: 10.3390/ijms20143380. Int J Mol Sci. 2019. PMID: 31295812 Free PMC article. Review.
-
Beneficial Effects of Flavonoids Against Parkinson's Disease.J Med Food. 2018 May;21(5):421-432. doi: 10.1089/jmf.2017.4078. Epub 2018 Feb 7. J Med Food. 2018. PMID: 29412767 Review.
-
Naringenin alleviates paraquat-induced dopaminergic neuronal loss in SH-SY5Y cells and a rat model of Parkinson's disease.Neuropharmacology. 2021 Dec 15;201:108831. doi: 10.1016/j.neuropharm.2021.108831. Epub 2021 Oct 13. Neuropharmacology. 2021. PMID: 34655599
-
Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features.Cent Nerv Syst Agents Med Chem. 2013 Mar;13(1):30-5. doi: 10.2174/1871524911313010005. Cent Nerv Syst Agents Med Chem. 2013. PMID: 23092407 Review.
Cited by
-
Gardenin A treatment attenuates inflammatory markers, synuclein pathology and deficits in tyrosine hydroxylase expression and improves cognitive and motor function in A53T-α-syn mice.Biomed Pharmacother. 2024 Apr;173:116370. doi: 10.1016/j.biopha.2024.116370. Epub 2024 Mar 8. Biomed Pharmacother. 2024. PMID: 38458012 Free PMC article.
References
-
- Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23(4–5):487–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

