Mapping the interplay between NK cells and HIV: therapeutic implications
- PMID: 36822173
- PMCID: PMC10043732
- DOI: 10.1093/jleuko/qiac007
Mapping the interplay between NK cells and HIV: therapeutic implications
Abstract
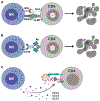
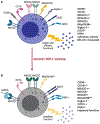
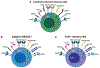
Although highly effective at durably suppressing plasma HIV-1 viremia, combination antiretroviral therapy (ART) treatment regimens do not eradicate the virus, which persists in long-lived CD4+ T cells. This latent viral reservoir serves as a source of plasma viral rebound following treatment interruption, thus requiring lifelong adherence to ART. Additionally, challenges remain related not only to access to therapy but also to a higher prevalence of comorbidities with an inflammatory etiology in treated HIV-1+ individuals, underscoring the need to explore therapeutic alternatives that achieve sustained virologic remission in the absence of ART. Natural killer (NK) cells are uniquely positioned to positively impact antiviral immunity, in part due to the pleiotropic nature of their effector functions, including the acquisition of memory-like features, and, therefore, hold great promise for transforming HIV-1 therapeutic modalities. In addition to defining the ability of NK cells to contribute to HIV-1 control, this review provides a basic immunologic understanding of the impact of HIV-1 infection and ART on the phenotypic and functional character of NK cells. We further delineate the qualities of "memory" NK cell populations, as well as the impact of HCMV on their induction and subsequent expansion in HIV-1 infection. We conclude by highlighting promising avenues for optimizing NK cell responses to improve HIV-1 control and effect a functional cure, including blockade of inhibitory NK receptors, TLR agonists to promote latency reversal and NK cell activation, CAR NK cells, BiKEs/TriKEs, and the role of HIV-1-specific bNAbs in NK cell-mediated ADCC activity against HIV-1-infected cells.
Keywords: HIV; adaptive NK cells; broadly neutralizing antibodies (bNAbs); dendritic cells (DCs); innate immune memory; memory NK cells.
© The Author(s) 2023. Published by Oxford University Press on behalf of Society for Leukocyte Biology.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures


Similar articles
-
Early antiretroviral therapy and its impact on natural killer cell dynamics in HIV-1 infected men who have sex with men: a cross-sectional pilot study evaluating the impact of early ART initiation on NK cell perturbation in HIV infection.Microbiol Spectr. 2024 Apr 2;12(4):e0357023. doi: 10.1128/spectrum.03570-23. Epub 2024 Feb 16. Microbiol Spectr. 2024. PMID: 38364104 Free PMC article.
-
A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2016 Apr 14;90(9):4441-4453. doi: 10.1128/JVI.00222-16. Print 2016 May. J Virol. 2016. PMID: 26889036 Free PMC article.
-
Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo.J Virol. 2018 May 29;92(12):e00235-18. doi: 10.1128/JVI.00235-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29593039 Free PMC article.
-
Antibodies and Antibody Derivatives: New Partners in HIV Eradication Strategies.Front Immunol. 2018 Oct 23;9:2429. doi: 10.3389/fimmu.2018.02429. eCollection 2018. Front Immunol. 2018. PMID: 30405624 Free PMC article. Review.
-
NK cell-based therapies for HIV infection: Investigating current advances and future possibilities.J Leukoc Biol. 2022 Apr;111(4):921-931. doi: 10.1002/JLB.5RU0821-412RR. Epub 2021 Oct 20. J Leukoc Biol. 2022. PMID: 34668588 Review.
Cited by
-
Pharmacological approaches to promote cell death of latent HIV reservoirs.Curr Opin HIV AIDS. 2024 Mar 1;19(2):56-61. doi: 10.1097/COH.0000000000000837. Epub 2023 Dec 20. Curr Opin HIV AIDS. 2024. PMID: 38169429 Free PMC article. Review.
-
Afucosylated broadly neutralizing antibodies enhance clearance of HIV-1 infected cells through cell-mediated killing.Commun Biol. 2024 Aug 9;7(1):964. doi: 10.1038/s42003-024-06659-8. Commun Biol. 2024. PMID: 39122901 Free PMC article.
-
Persistence of a Skewed Repertoire of NK Cells in People with HIV-1 on Long-Term Antiretroviral Therapy.J Immunol. 2024 May 15;212(10):1564-1578. doi: 10.4049/jimmunol.2300672. J Immunol. 2024. PMID: 38551350
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

