Monoclonal antibody targeting a novel linear epitope on nucleoprotein confers pan-reactivity to influenza A virus
- PMID: 36820898
- PMCID: PMC9947902
- DOI: 10.1007/s00253-023-12433-3
Monoclonal antibody targeting a novel linear epitope on nucleoprotein confers pan-reactivity to influenza A virus
Abstract
Nucleoprotein (NP) functions crucially in the replicative cycle of influenza A virus (IAV) via forming the ribonucleoprotein complex together with PB2, PB1, and PA proteins. As its high conservation, NP ranks one of the hot targets for design of universal diagnostic reagents and antiviral drugs for IAV. Here, we report an anti-NP murine monoclonal antibody (mAb) 5F10 prepared from traditional lymphocyte hybridoma technique with the immunogen of a clade 2.3.4.4 H5N1 subtype avian influenza virus. The specificity of mAb 5F10 to NP protein was confirmed by immunofluorescence assay and western blotting, and the mAb 5F10 could be used in immunoprecipitation and immunohistochemistry assays. Importantly, mAb 5F10 possessed broad-spectrum reactivity against H1~H11 subtypes of avian influenza viruses, including various HA clades of H5Nx subtype. In addition, mAb 5F10 also showed good affinity with H1N1 and H3N2 subtype influenza viruses of swine and human origin. Furthermore, the recognized antigenic epitope of mAb 5F10 was identified to consist of the conserved amino acid motif 81EHPSA85 in the second flexible loop region of NP protein through screening the phage display peptide library. Collectively, the mAb 5F10 which recognizes the novel universal NP linear B-cell epitope of IAV with diverse origins and subtypes will be a powerful tool for NP protein-based structural, functional, and mechanistic studies, as well as the development of detection methods and universal vaccines for IAV. KEY POINTS: • A broad-spectrum mAb against various subtypes and sources of IAV was developed • The mAb possessed good reactivity in IFA, western blot, IP, and IHC assays • The mAb targeted a novel conserved linear B-cell epitope involving 81EHPSA85 on NP protein.
Keywords: Influenza virus; Linear B-cell epitope; Monoclonal antibody; Nucleoprotein; Universal reactivity.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests
Figures

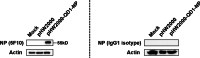


Similar articles
-
Development and application of monoclonal antibodies against avian influenza virus nucleoprotein.J Virol Methods. 2008 Feb;147(2):265-74. doi: 10.1016/j.jviromet.2007.09.016. Epub 2007 Nov 19. J Virol Methods. 2008. PMID: 18006085
-
Alternative recognition of the conserved stem epitope in influenza A virus hemagglutinin by a VH3-30-encoded heterosubtypic antibody.J Virol. 2014 Jun;88(12):7083-92. doi: 10.1128/JVI.00178-14. Epub 2014 Apr 9. J Virol. 2014. PMID: 24719426 Free PMC article.
-
Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells.PLoS One. 2008;3(12):e3942. doi: 10.1371/journal.pone.0003942. Epub 2008 Dec 16. PLoS One. 2008. PMID: 19079604 Free PMC article.
-
Development and biochemical characterization of the monoclonal antibodies for specific detection of the emerging H5N8 and H5Nx avian influenza virus hemagglutinins.Appl Microbiol Biotechnol. 2021 Jan;105(1):235-245. doi: 10.1007/s00253-020-11035-7. Epub 2020 Nov 27. Appl Microbiol Biotechnol. 2021. PMID: 33245391
-
Identification of a highly conserved and surface exposed B-cell epitope on the nucleoprotein of influenza A virus.J Med Virol. 2014 Jun;86(6):995-1002. doi: 10.1002/jmv.23812. Epub 2013 Oct 17. J Med Virol. 2014. PMID: 24136709
References
-
- Asdaq SMB, Rabbani SI, Alkahtani M, Aldohyan MM, Alabdulsalam AM, Alshammari MS, Alajlan SA, Binrokan A, Mohzari Y, Alrashed A, Alshammari MK, Imran M, Nayeem N. A patent review on the therapeutic application of monoclonal antibodies in COVID-19. Int J Mol Sci. 2021;22(21):11953. doi: 10.3390/ijms222111953. - DOI - PMC - PubMed
-
- Bodewes R, Geelhoed-Mieras MM, Wrammert J, Ahmed R, Wilson PC, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. In vitro assessment of the immunological significance of a human monoclonal antibody directed to the influenza a virus nucleoprotein. Clin Vaccine Immunol. 2013;20(8):1333–1337. doi: 10.1128/CVI.00339-13. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

