A systematic review of diagnostic, prognostic, and risk blood and urine biomarkers of transplant-associated thrombotic microangiopathy
- PMID: 36818475
- PMCID: PMC9933706
- DOI: 10.3389/fimmu.2022.1064203
A systematic review of diagnostic, prognostic, and risk blood and urine biomarkers of transplant-associated thrombotic microangiopathy
Abstract
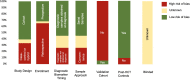
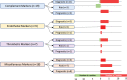
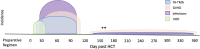
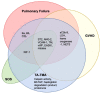
Transplant-associated thrombotic microangiopathy (TA-TMA) is an increasingly recognized complication of allogeneic and autologous hematopoietic cellular therapy (HCT), associated with significant morbidity and mortality. Although the central drivers of the disease are thought to be endothelial damage and complement activation, no specific diagnostic biomarkers have been identified. TA-TMA is typically diagnosed using criteria comprised of non-specific clinical and laboratory features. Some patients will have a self-remitting course, but more than half develop multi-organ dysfunction or die, making prognostic biomarkers critical. Prevention of TA-TMA, an approach central to other HCT complications such as graft-versus-host disease, is largely untested in part due to a lack of identified early high-risk biomarkers. We conducted a systematic review to summarize the diagnostic, early risk, and prognostic biomarkers of TA-TMA. We screened the titles and abstracts of 1524 citations. After screening out duplications, we read the abstracts of 979 papers and fully reviewed 132 full-text publications. Thirty-one publications fulfilled the inclusion criteria of more than five patients with TA-TMA and a reported measure of association with diagnosis, prognosis, or risk of later development of the disease. Fourteen studies (45%) were with adults, 12 (39%) were with children <18 years old, three included both children and adults, and two did not report age. There were 53 biomarker or biomarker signature entries, and a total of 27 unique biomarkers. Only four biomarkers reported sensitivity and specificity. The single biomarker with the most robust data was sC5b-9, which conferred diagnostic, prognostic, and risk implications. Studies of combinations of biomarkers were rare. No meta-analyses were performed because of significant heterogeneity between studies. The limitations of studies included small sample size, study designs with a high risk of bias (i.e., case-control), the timing of sample collection, and the selection of controls. Furthermore, only two (6%) studies included a training and validation cohort. Cut-off points are needed to stratify groups, as most biomarkers do not have normal values, or normal values cannot be assumed in the HCT setting. In the future, multi-institutional, collaborative efforts are needed to perform rigorously designed, prospective studies with serially enrolled patients, with samples collected at the time of TA-TMA diagnosis, careful selection of controls, and validation of selected biomarkers and cut-off points in a separate cohort.
Keywords: NETs (neutrophil extracellular traps); biomarkers; complement activation; endothelial activation; sC5b-9 (serum complement membrane attack complex); transplant associated thrombotic microangiopathy.
Copyright © 2023 Schoettler, Bhatt and Vasu.
Conflict of interest statement
SV is a consultant for Omeros. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Early Increase in Complement Terminal Pathway Activation Marker sC5b-9 Is Predictive for the Development of Thrombotic Microangiopathy after Stem Cell Transplantation.Biol Blood Marrow Transplant. 2018 May;24(5):989-996. doi: 10.1016/j.bbmt.2018.01.009. Epub 2018 Jan 12. Biol Blood Marrow Transplant. 2018. PMID: 29339271
-
Transplant-associated thrombotic microangiopathy: Incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation.Clin Transplant. 2018 Sep;32(9):e13371. doi: 10.1111/ctr.13371. Epub 2018 Aug 20. Clin Transplant. 2018. PMID: 30080283
-
Real-World Application of Recently Proposed ASTCT/CIBMTR/EBMT/APBMT Consensus Risk Stratification for Transplantation-Associated Thrombotic Microangiopathy in Children.Transplant Cell Ther. 2024 Sep;30(9):929.e1-929.e6. doi: 10.1016/j.jtct.2024.06.017. Epub 2024 Jun 25. Transplant Cell Ther. 2024. PMID: 38936547
-
Transplant-Associated Thrombotic Microangiopathy in Pediatric Hematopoietic Cell Transplant Recipients: A Practical Approach to Diagnosis and Management.Front Pediatr. 2019 Apr 9;7:133. doi: 10.3389/fped.2019.00133. eCollection 2019. Front Pediatr. 2019. PMID: 31024873 Free PMC article. Review.
-
Emerging therapeutic and preventive approaches to transplant-associated thrombotic microangiopathy.Curr Opin Hematol. 2021 Nov 1;28(6):408-416. doi: 10.1097/MOH.0000000000000687. Curr Opin Hematol. 2021. PMID: 34534983 Free PMC article. Review.
Cited by
-
Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) and Growth Differentiation Factor-15 (GDF-15) Levels Are Significantly Associated with Endothelial Injury Indices in Adult Allogeneic Hematopoietic Cell Transplantation Recipients.Int J Mol Sci. 2023 Dec 23;25(1):231. doi: 10.3390/ijms25010231. Int J Mol Sci. 2023. PMID: 38203404 Free PMC article.
References
-
- Gavriilaki E, Sakellari I, Batsis I, Mallouri D, Bousiou Z, Vardi A, et al. . Transplant-associated thrombotic microangiopathy: Incidence, prognostic factors, morbidity, and mortality in allogeneic hematopoietic cell transplantation. Clin Transplant. (2018) 32(9):e13371. doi: 10.1111/ctr.13371 - DOI - PubMed
-
- Jodele S, Dandoy CE, Myers K, Wallace G, Lane A, Teusink-Cross A, et al. . High-dose Carboplatin/Etoposide/Melphalan increases risk of thrombotic microangiopathy and organ injury after autologous stem cell transplantation in patients with neuroblastoma. Bone Marrow Transplant. (2018). doi: 10.1038/s41409-018-0159-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

