Angiotensin II increases respiratory rhythmic activity in the preBötzinger complex without inducing astroglial calcium signaling
- PMID: 36816850
- PMCID: PMC9932970
- DOI: 10.3389/fncel.2023.1111263
Angiotensin II increases respiratory rhythmic activity in the preBötzinger complex without inducing astroglial calcium signaling
Abstract
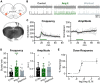
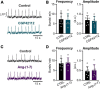
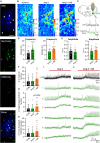
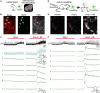
Angiotensin II (Ang II) is the primary modulator of the renin-angiotensin system and has been widely studied for its effect on the cardiovascular system. While a few studies have also indicated an involvement of Ang II in the regulation of breathing, very little is known in this regard and its effect on brainstem respiratory regions such as the preBötzinger complex (preBötC), the kernel for inspiratory rhythm generation, has not been investigated yet. This study reports that Ang II temporarily increases phrenic nerve activity in the working heart-brainstem preparation, indicating higher central respiratory drive. Previous studies have shown that the carotid body is involved in mediating this effect and we revealed that the preBötC also plays a part, using acute slices of the brainstem. It appears that Ang II is increasing the respiratory drive in an AT1R-dependent manner by optimizing the interaction of inhibitory and excitatory neurons of the preBötC. Thus, Ang II-mediated effects on the preBötC are potentially involved in dysregulating breathing in patients with acute lung injury.
Keywords: angiotensin II; astrocytes; preBötzinger complex (preBötC); respiration; respiratory activity.
Copyright © 2023 Tacke, Bischoff, Harb, Vafadari and Hülsmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dendritic A-Current in Rhythmically Active PreBötzinger Complex Neurons in Organotypic Cultures from Newborn Mice.J Neurosci. 2018 Mar 21;38(12):3039-3049. doi: 10.1523/JNEUROSCI.3342-17.2018. Epub 2018 Feb 19. J Neurosci. 2018. PMID: 29459371 Free PMC article.
-
Selective transduction and photoinhibition of pre-Bötzinger complex neurons that project to the facial nucleus in rats affects nasofacial activity.Elife. 2023 Sep 29;12:e85398. doi: 10.7554/eLife.85398. Elife. 2023. PMID: 37772793 Free PMC article.
-
Efferent projections of excitatory and inhibitory preBötzinger Complex neurons.J Comp Neurol. 2018 Jun 1;526(8):1389-1402. doi: 10.1002/cne.24415. Epub 2018 Mar 9. J Comp Neurol. 2018. PMID: 29473167 Free PMC article.
-
Pre-Bötzinger complex: Generation and modulation of respiratory rhythm.Neurologia (Engl Ed). 2019 Sep;34(7):461-468. doi: 10.1016/j.nrl.2016.05.011. Epub 2016 Jul 18. Neurologia (Engl Ed). 2019. PMID: 27443242 Review. English, Spanish.
-
Respiratory Rhythm Generation: The Whole Is Greater Than the Sum of the Parts.Adv Exp Med Biol. 2017;1015:147-161. doi: 10.1007/978-3-319-62817-2_9. Adv Exp Med Biol. 2017. PMID: 29080026 Review.
Cited by
-
Fiber optical imaging of astroglial calcium signaling in the respiratory network in the working heart brainstem preparation.Front Physiol. 2023 Aug 24;14:1237376. doi: 10.3389/fphys.2023.1237376. eCollection 2023. Front Physiol. 2023. PMID: 37693007 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

