Persistent hematopoietic polyclonality after lentivirus-mediated gene therapy for Fabry disease
- PMID: 36816757
- PMCID: PMC9932294
- DOI: 10.1016/j.omtm.2023.01.003
Persistent hematopoietic polyclonality after lentivirus-mediated gene therapy for Fabry disease
Abstract
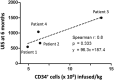
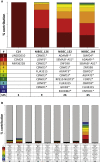
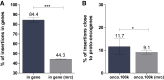
The safety and efficacy of lentivirus-mediated gene therapy was recently demonstrated in five male patients with Fabry disease-a rare X-linked lysosomal storage disorder caused by GLA gene mutations that result in multiple end-organ complications. To evaluate the risks of clonal dominance and leukemogenesis, which have been reported in multiple gene therapy trials, we conducted a comprehensive DNA insertion site analysis of peripheral blood samples from the five patients in our gene therapy trial. We found that patients had a polyclonal integration site spectrum and did not find evidence of a dominant clone in any patient. Although we identified vector integrations near proto-oncogenes, these had low percentages of contributions to the overall pool of integrations and did not persist over time. Overall, we show that our trial of lentivirus-mediated gene therapy for Fabry disease did not lead to hematopoietic clonal dominance and likely did not elevate the risk of leukemogenic transformation.
Keywords: Fabry disease; clinical trial; gene therapy; lentiviral integration; lentiviral safety.
© 2023 The Authors.
Conflict of interest statement
D.L.B. was partially paid from a sponsored research agreement from AVROBIO, Inc. C.A.-B. has received a service contract and honoraria for biomarker analysis with AVROBIO, Inc. and a grant from CIHR. C.F.M. has received grants, personal fees, and non-financial support from Takeda Pharmaceuticals (previously Shire HGT); grants, personal fees, and non-financial support from Sanofi-Genzyme; and non-financial support from Amicus Therapeutics. A. Khan received grants, consulting fees, revenue distribution agreement, speaker fees, and travel support with AVROBIO, Inc., as well as has a revenue distribution agreement with University Health Network regarding gene therapy using technology from this work. M.L.W. has received research grants, consulting fees, speaker fees, and travel support from Amicus Therapeutics, Protalix, Sanofi-Genzyme, and Takeda and has a revenue distribution agreement with University Health Network regarding gene therapy using technology from this work. C.A.R. has the following financial relationships to disclose: the Biochemical Genetics clinical diagnostic laboratory at his home institution is contracted by AVROBIO, Inc., to assay enzymes on a fee-for--service basis. He is the laboratory director but receives no personal compensation. J.A.M. is on the scientific advisory board of Rapa Therapeutics; has received honoraria from Sanofi Genzyme and Shire; is a co-founder and shareholder of AVROBIO, Inc.; and has received grants from Canadian Institutes of Health Research and Kidney Foundation of Canada and AVROBIO, Inc. A. Keating has received a consultancy fee from AVROBIO, Inc., unrelated to this study. A.S. and M.R. have a service contract and received consultancy fees from AVROBIO, Inc., unrelated to this study.
Figures

Similar articles
-
Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy.Methods Enzymol. 2012;507:187-98. doi: 10.1016/B978-0-12-386509-0.00010-7. Methods Enzymol. 2012. PMID: 22365775 Clinical Trial.
-
Quantitative shearing linear amplification polymerase chain reaction: an improved method for quantifying lentiviral vector insertion sites in transplanted hematopoietic cell systems.Hum Gene Ther Methods. 2015 Feb;26(1):4-12. doi: 10.1089/hgtb.2014.122. Epub 2015 Feb 5. Hum Gene Ther Methods. 2015. PMID: 25545666 Free PMC article.
-
Mouse transplant models for evaluating the oncogenic risk of a self-inactivating XSCID lentiviral vector.PLoS One. 2013 Apr 23;8(4):e62333. doi: 10.1371/journal.pone.0062333. Print 2013. PLoS One. 2013. PMID: 23626802 Free PMC article.
-
The MDS1-EVI1 gene complex as a retrovirus integration site: impact on behavior of hematopoietic cells and implications for gene therapy.Mol Ther. 2008 Mar;16(3):439-49. doi: 10.1038/sj.mt.6300372. Epub 2008 Jan 29. Mol Ther. 2008. PMID: 18227842 Review.
-
Gene therapy for Fabry disease.J Inherit Metab Dis. 2001;24 Suppl 2:25-41; discussion 11-2. doi: 10.1023/a:1012455421014. J Inherit Metab Dis. 2001. PMID: 11758676 Review.
Cited by
-
Preclinical lentiviral hematopoietic stem cell gene therapy corrects Pompe disease-related muscle and neurological manifestations.Mol Ther. 2024 Nov 6;32(11):3847-3864. doi: 10.1016/j.ymthe.2024.09.024. Epub 2024 Sep 17. Mol Ther. 2024. PMID: 39295144
References
-
- Zarate Y.A., Hopkin R.J. Fabry's disease. Lancet. 2008;372:1427–1435. - PubMed
-
- Ortiz A., Germain D.P., Desnick R.J., Politei J., Mauer M., Burlina A., Eng C., Hopkin R.J., Laney D., Linhart A., et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018;123:416–427. - PubMed
-
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
LinkOut - more resources
Full Text Sources

