The impact of stress and anesthesia on animal models of infectious disease
- PMID: 36816193
- PMCID: PMC9933909
- DOI: 10.3389/fvets.2023.1086003
The impact of stress and anesthesia on animal models of infectious disease
Abstract
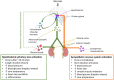
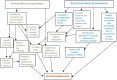
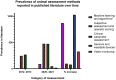
Stress and general anesthesia have an impact on the functional response of the organism due to the detrimental effects on cardiovascular, immunological, and metabolic function, which could limit the organism's response to an infectious event. Animal studies have formed an essential step in understanding and mitigating infectious diseases, as the complexities of physiology and immunity cannot yet be replicated in vivo. Using animals in research continues to come under increasing societal scrutiny, and it is therefore crucial that the welfare of animals used in disease research is optimized to meet both societal expectations and improve scientific outcomes. Everyday management and procedures in animal studies are known to cause stress, which can not only cause poorer welfare outcomes, but also introduces variables in disease studies. Whilst general anesthesia is necessary at times to reduce stress and enhance animal welfare in disease research, evidence of physiological and immunological disruption caused by general anesthesia is increasing. To better understand and quantify the effects of stress and anesthesia on disease study and welfare outcomes, utilizing the most appropriate animal monitoring strategies is imperative. This article aims to analyze recent scientific evidence about the impact of stress and anesthesia as uncontrolled variables, as well as reviewing monitoring strategies and technologies in animal models during infectious diseases.
Keywords: animal immunity; animal models of disease; animal monitoring; impacts of anesthesia; impacts of stress; infectious disease research; laboratory animal welfare; surgical stress.
Copyright © 2023 Layton, Layton, Beggs, Fisher, Mansell and Stanger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Pain and Laboratory Animals: Publication Practices for Better Data Reproducibility and Better Animal Welfare.PLoS One. 2016 May 12;11(5):e0155001. doi: 10.1371/journal.pone.0155001. eCollection 2016. PLoS One. 2016. PMID: 27171143 Free PMC article.
-
Culture of Care: Organizational Responsibilities.In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. In: Weichbrod RH, Thompson GA, Norton JN, editors. Management of Animal Care and Use Programs in Research, Education, and Testing. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. PMID: 29787190 Free Books & Documents. Review.
-
A critical review of anaesthetised animal models and alternatives for military research, testing and training, with a focus on blast damage, haemorrhage and resuscitation.Altern Lab Anim. 2013 Nov;41(5):385-415. doi: 10.1177/026119291304100508. Altern Lab Anim. 2013. PMID: 24329746 Review.
-
Anesthesia and Monitoring of Animals During MRI Studies.Methods Mol Biol. 2018;1718:423-439. doi: 10.1007/978-1-4939-7531-0_25. Methods Mol Biol. 2018. PMID: 29341023
-
Linking stress and immunity: Immunoglobulin A as a non-invasive physiological biomarker in animal welfare studies.Horm Behav. 2018 Jun;102:55-68. doi: 10.1016/j.yhbeh.2018.04.011. Epub 2018 May 10. Horm Behav. 2018. PMID: 29705025 Review.
Cited by
-
Artificial Feeding Systems for Vector-Borne Disease Studies.Biology (Basel). 2024 Mar 15;13(3):188. doi: 10.3390/biology13030188. Biology (Basel). 2024. PMID: 38534457 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

