Motilin and its receptor are expressed in the dorsal horn in a rat model of acute incisional pain: Intrathecal motilin injection alleviates pain behaviors
- PMID: 36816129
- PMCID: PMC9932669
- DOI: 10.3389/fnins.2023.1104862
Motilin and its receptor are expressed in the dorsal horn in a rat model of acute incisional pain: Intrathecal motilin injection alleviates pain behaviors
Abstract
Aims: To observe the effects of intrathecal administration of motilin on pain behavior and expression of motilin (MTL)/motilin receptor (MTLR) in the spinal cord of a rat model of acute incisional pain.
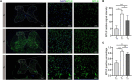
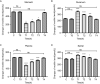
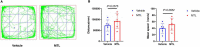
Methods: An incisional pain model was established in rats using a unilateral plantar incision. The rats were also injected intrathecally with 1, 5, or 25 μg of motilin. The mechanical withdrawal threshold (MWT) and thermal withdrawal latency (TWL) were determined. MTL/MTLR expression in the spinal cord was detected by western blotting and immunofluorescence. The expression of MTL in the spinal cord, stomach, duodenum, and plasma was determined by enzyme-linked immunosorbent assay (ELISA).
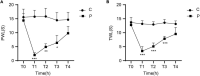
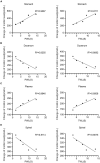
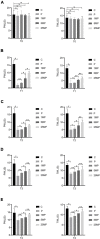
Results: Motilin/motilin receptor were detected in the spinal cord. Spinal cord MTL/MTLR expression peaks at 2 h after modeling (P < 0.05) and start to decrease at 24 h (P < 0.05) to almost reach baseline levels at 72 h. The changes in gastric, duodenal, plasma, and spinal cord motilin levels correlated with MWT and TWL (all R 2 > 0.82). The intrathecal injection of 1, 5, or 25 μg of motilin could increase the pain threshold of rats with incisional pain within 72 h in a dose-dependent manner.
Conclusion: This study showed for the first time that MTL/MTLR are expressed in rats' spinal dorsal horn. Acute pain increased MTL/MTLR expression in the spinal dorsal horn. Also, for the first time, this study showed that motilin intrathecal injection alleviates pain in rat models of acute incisional pain. These results suggest that MTL/MTLR could be a novel target for the management of acute pain.
Keywords: acute pain; animal model; motilin; motilin receptors; spinal cord.
Copyright © 2023 Zhang, Zhao, Hu, Wang, Chen, Wang and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

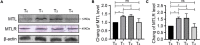
Similar articles
-
Effects of intrathecal opioids combined with low-dose naloxone on motilin and its receptor in a rat model of postoperative pain.Life Sci. 2014 May 17;103(2):88-94. doi: 10.1016/j.lfs.2014.03.032. Epub 2014 Apr 13. Life Sci. 2014. PMID: 24727237
-
[Analgesic effect and mechanism of electroacupuncture on SNI rats based on microglia-BDNF-neuron signal].Zhongguo Zhen Jiu. 2022 Sep 12;42(9):1029-36. doi: 10.13703/j.0255-2930.20210617-0004. Zhongguo Zhen Jiu. 2022. PMID: 36075600 Chinese.
-
[Melatonin receptor 2 mediated spinal dorsal horn astrocytes IL-17 inhibition contributes to electroacupuncture analgesia in neuropathic pain rats].Zhen Ci Yan Jiu. 2021 Jul 25;46(7):562-9. doi: 10.13702/j.1000-0607.201158. Zhen Ci Yan Jiu. 2021. PMID: 34369675 Chinese.
-
[Expression of connexin 43 in spinal cord dorsal horn of rats with acute incisional pain].Nan Fang Yi Ke Da Xue Xue Bao. 2015 Mar;35(3):387-9, 396. Nan Fang Yi Ke Da Xue Xue Bao. 2015. PMID: 25818786 Chinese.
-
Upregulation of spinal glucose-dependent insulinotropic polypeptide receptor induces membrane translocation of PKCγ and synaptic target of AMPA receptor GluR1 subunits in dorsal horns in a rat model of incisional pain.Neurochem Int. 2020 Mar;134:104651. doi: 10.1016/j.neuint.2019.104651. Epub 2019 Dec 20. Neurochem Int. 2020. PMID: 31870892
Cited by
-
Motilin fluctuations in healthy volunteers determined by liquid chromatography mass spectrometry.Front Endocrinol (Lausanne). 2024 Mar 13;15:1348146. doi: 10.3389/fendo.2024.1348146. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38544692 Free PMC article.
-
The influence of herbal medicine on serum motilin and its effect on human and animal model: a systematic review.Front Pharmacol. 2023 Dec 14;14:1286333. doi: 10.3389/fphar.2023.1286333. eCollection 2023. Front Pharmacol. 2023. PMID: 38161695 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

