First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring
- PMID: 36815245
- PMCID: PMC9970198
- DOI: 10.1080/10717544.2023.2180113
First-in-human study to assess the pharmacokinetics, tolerability, and safety of single-dose oxybutynin hydrochloride administered via a microprocessor-controlled intravaginal ring
Abstract
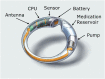
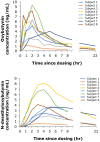
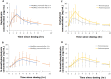
Polymeric drug-releasing vaginal rings are useful for both local and systemic administration of drugs via the intravaginal route. Typically, they provide continuous sustained or controlled release of drug(s) over extended time periods, thereby avoiding overdose and improving adherence. This first-in-human study (EudraCT number: 2020-0050044-30) evaluated the pharmacokinetics, safety, and tolerability of a single dose of oxybutynin administered by a novel microprocessor-controlled vaginal ring (MedRing). Eight healthy female subjects received an electronically controlled single intravaginal dose of 3 mg oxybutynin hydrochloride (100 mg/mL) dissolved in 1:1 water/propylene glycol administered via MedRing. Following dosing, MedRing was kept in situ for up to 6 h. Blood samples were collected 1 h prior to oxybutynin dosing and subsequently at regular intervals post-dose for the assessment of plasma concentrations of oxybutynin and its active metabolite N-desethyloxybutynin. The results showed that MedRing efficiently administered oxybutynin via the intravaginal route, resulting in plasma oxybutynin levels comparable to orally administered oxybutynin. The mean ± standard deviation pharmacokinetic parameters for oxybutynin were Cmax 5.4 ± 2.7 ng/mL, AUCinf 34.9 ± 17.4 h ng/mL, t1/2 8.5 ± 3.5 h and for N-desethyloxybutynin were Cmax 3.9 ± 2.5 ng/mL, AUCinf 51.1 ± 43.1 h ng/mL, t1/2 7.7 ± 5.9 h. No serious adverse events were reported. The study demonstrates that intravaginal administration of oxybutynin hydrochloride using the MedRing device was well tolerated.
Keywords: N-desethyloxybutynin; Vaginal ring; urinary incontinence.
Conflict of interest statement
Karl Malcolm, Naomi Klarenbeek, Lisa Pagan, Bertine Huisman, Rik Stuurman, and Michiel van Esdonk declare no conflict of interest. Willem de Laat, Victor Nickolson, and Maarten Wiegerinck are employed by LiGalli.
Figures
Similar articles
-
Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate-release oxybutynin.J Clin Pharmacol. 1999 Mar;39(3):289-96. J Clin Pharmacol. 1999. PMID: 10073329 Clinical Trial.
-
Pharmacokinetics, efficacy, and safety of intravesical formulation of oxybutynin in patients with detrusor overactivity.Scand J Urol Nephrol. 2002 Feb;36(1):18-24. doi: 10.1080/003655902317259319. Scand J Urol Nephrol. 2002. PMID: 12002352 Clinical Trial.
-
Pharmacokinetics of oxybutynin chloride topical gel: effects of application site, baths, sunscreen and person-to-person transference.Clin Drug Investig. 2011;31(8):559-571. doi: 10.2165/11588990-000000000-00000. Clin Drug Investig. 2011. PMID: 21721591 Clinical Trial.
-
An extended-release formulation of oxybutynin chloride for the treatment of overactive urinary bladder.Clin Ther. 1999 Apr;21(4):634-42. doi: 10.1016/S0149-2918(00)88316-2. Clin Ther. 1999. PMID: 10363730 Review.
-
Oxybutynin topical and transdermal formulations: an update.Drugs Today (Barc). 2010 Jun;46(6):417-25. doi: 10.1358/dot.2010.46.6.1487750. Drugs Today (Barc). 2010. PMID: 20571610 Review.
References
-
- Ahrendt HJ, Nisand I, Bastianelli C, et al. (2006). Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing, compared with an oral contraceptive containing 30 microg of ethinyl estradiol and 3 mg of drospirenone. Contraception 74:451–8. - PubMed
-
- Alexander NJ, Baker E, Kaptein M, et al. (2004). Why consider vaginal drug administration?. Fertil Steril 82:1–12. - PubMed
-
- Algorta J, Diaz M, de Benito R, et al. (2017). Pharmacokinetic bioequivalence, safety and acceptability of Ornibel(®), a new polymer composition contraceptive vaginal ring (etonogestrel/ethinylestradiol 11.00/3.474 mg) compared with Nuvaring(®) (etonogestrel/ethinylestradiol 11.7/2.7 mg). Eur J Contracept Reprod Health Care 22:429–38. - PubMed
-
- Appell RA, Chancellor MB, Zobrist RH, et al. (2003). Pharmacokinetics, metabolism, and saliva output during transdermal and extended-release oral oxybutynin administration in healthy subjects. Mayo Clin Proc 78:696–702. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
