Postnatal oogenesis leads to an exceptionally large ovarian reserve in naked mole-rats
- PMID: 36810851
- PMCID: PMC9944903
- DOI: 10.1038/s41467-023-36284-8
Postnatal oogenesis leads to an exceptionally large ovarian reserve in naked mole-rats
Abstract
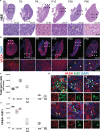
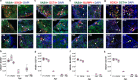
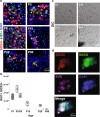
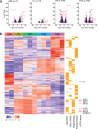
In the long-lived naked mole-rat (NMR), the entire process of oogenesis occurs postnatally. Germ cell numbers increase significantly in NMRs between postnatal days 5 (P5) and P8, and germs cells positive for proliferation markers (Ki-67, pHH3) are present at least until P90. Using pluripotency markers (SOX2 and OCT4) and the primordial germ cell (PGC) marker BLIMP1, we show that PGCs persist up to P90 alongside germ cells in all stages of female differentiation and undergo mitosis both in vivo and in vitro. We identified VASA+ SOX2+ cells at 6 months and at 3-years in subordinate and reproductively activated females. Reproductive activation was associated with proliferation of VASA+ SOX2+ cells. Collectively, our results suggest that highly desynchronized germ cell development and the maintenance of a small population of PGCs that can expand upon reproductive activation are unique strategies that could help to maintain the NMR's ovarian reserve for its 30-year reproductive lifespan.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Germ cell nests in adult ovaries and an unusually large ovarian reserve in the naked mole-rat.Reproduction. 2021 Jan;161(1):89-98. doi: 10.1530/REP-20-0304. Reproduction. 2021. PMID: 33151901 Free PMC article.
-
Characterization of the Postnatal Naked Mole-Rat Ovary: From Primordial Germ Cells to Meiotic Prophase I Oocytes.Methods Mol Biol. 2023;2677:185-201. doi: 10.1007/978-1-0716-3259-8_11. Methods Mol Biol. 2023. PMID: 37464243
-
The infant and pubertal human ovary: Balbiani's body-associated VASA expression, immunohistochemical detection of apoptosis-related BCL2 and BAX proteins, and DNA fragmentation.Hum Reprod. 2013 Mar;28(3):698-706. doi: 10.1093/humrep/des453. Epub 2013 Jan 12. Hum Reprod. 2013. PMID: 23315064
-
How Is the Number of Primordial Follicles in the Ovarian Reserve Established?Biol Reprod. 2015 Nov;93(5):111. doi: 10.1095/biolreprod.115.133652. Epub 2015 Sep 30. Biol Reprod. 2015. PMID: 26423124 Review.
-
The safety of VASApos presumptive adult ovarian stem cells.Reprod Biomed Online. 2021 Oct;43(4):587-597. doi: 10.1016/j.rbmo.2021.06.017. Epub 2021 Jun 26. Reprod Biomed Online. 2021. PMID: 34474974 Review.
Cited by
-
Culture of the Intact Postnatal Naked Mole-Rat Ovary: From Meiotic Prophase to Single-Cell RNASeq.Methods Mol Biol. 2024;2818:179-194. doi: 10.1007/978-1-0716-3906-1_12. Methods Mol Biol. 2024. PMID: 39126475
-
In vitro production of naked mole-rats' blastocysts from non-breeding females using in vitro maturation and intracytoplasmic sperm injection.Sci Rep. 2023 Dec 15;13(1):22355. doi: 10.1038/s41598-023-49661-6. Sci Rep. 2023. PMID: 38102304 Free PMC article.
-
The journey of a generation: advances and promises in the study of primordial germ cell migration.Development. 2024 Apr 1;151(7):dev201102. doi: 10.1242/dev.201102. Epub 2024 Apr 12. Development. 2024. PMID: 38607588 Free PMC article.
-
Loss of function of male-specific lethal 3 (Msl3) does not affect spermatogenesis in rodents.Dev Dyn. 2024 May;253(5):453-466. doi: 10.1002/dvdy.669. Epub 2023 Oct 17. Dev Dyn. 2024. PMID: 37847071
-
Cellular senescence induction leads to progressive cell death via the INK4a-RB pathway in naked mole-rats.EMBO J. 2023 Aug 15;42(16):e111133. doi: 10.15252/embj.2022111133. Epub 2023 Jul 11. EMBO J. 2023. PMID: 37431790 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

