Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response
- PMID: 36810544
- PMCID: PMC9944956
- DOI: 10.3390/diseases11010030
Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response
Abstract
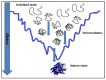
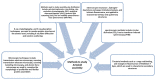
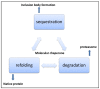
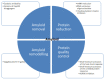
Proteins are central to life functions. Alterations in the structure of proteins are reflected in their function. Misfolded proteins and their aggregates present a significant risk to the cell. Cells have a diverse but integrated network of protection mechanisms. Streams of misfolded proteins that cells are continuously exposed to must be continually monitored by an elaborated network of molecular chaperones and protein degradation factors to control and contain protein misfolding problems. Aggregation inhibition properties of small molecules such as polyphenols are important as they possess other beneficial properties such as antioxidative, anti-inflammatory, and pro-autophagic properties and help neuroprotection. A candidate with such desired features is important for any possible treatment development for protein aggregation diseases. There is a need to study the protein misfolding phenomenon so that we can treat some of the worst kinds of human ailments related to protein misfolding and aggregation.
Keywords: aggregation inhibition; amyloid diseases; inflammation; neurodegeneration; protein aggregation.
Conflict of interest statement
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Comprehensive Perspective Towards the Management of Proteinopathies by Elucidating Protein Misfolding and Aggregation.CNS Neurol Disord Drug Targets. 2024;23(2):153-180. doi: 10.2174/1871527322666230306085937. CNS Neurol Disord Drug Targets. 2024. PMID: 36876837
-
Protein Quality Control by Molecular Chaperones in Neurodegeneration.Front Neurosci. 2017 Apr 6;11:185. doi: 10.3389/fnins.2017.00185. eCollection 2017. Front Neurosci. 2017. PMID: 28428740 Free PMC article. Review.
-
Mechanistic Insights into the Role of Molecular Chaperones in Protein Misfolding Diseases: From Molecular Recognition to Amyloid Disassembly.Int J Mol Sci. 2020 Dec 2;21(23):9186. doi: 10.3390/ijms21239186. Int J Mol Sci. 2020. PMID: 33276458 Free PMC article. Review.
-
Molecular pathogenesis of protein misfolding diseases: pathological molecular environments versus quality control systems against misfolded proteins.J Biochem. 2009 Dec;146(6):751-6. doi: 10.1093/jb/mvp119. Epub 2009 Jul 30. J Biochem. 2009. PMID: 19643812 Review.
-
Distinct role of hydration water in protein misfolding and aggregation revealed by fluctuating thermodynamics analysis.Acc Chem Res. 2015 Apr 21;48(4):956-65. doi: 10.1021/acs.accounts.5b00032. Epub 2015 Apr 6. Acc Chem Res. 2015. PMID: 25844814 Review.
Cited by
-
Could Targeting NPM1c+ Misfolding Be a Promising Strategy for Combating Acute Myeloid Leukemia?Int J Mol Sci. 2024 Jan 9;25(2):811. doi: 10.3390/ijms25020811. Int J Mol Sci. 2024. PMID: 38255885 Free PMC article. Review.
-
KCNH6 channel promotes insulin exocytosis via interaction with Munc18-1 independent of electrophysiological processes.Cell Mol Life Sci. 2024 Feb 13;81(1):86. doi: 10.1007/s00018-024-05134-1. Cell Mol Life Sci. 2024. PMID: 38349432 Free PMC article.
-
Anti-Aggregative and Protective Effects of Vicenin-2 on Heat and Oxidative Stress-Induced Damage on Protein Structures.Int J Mol Sci. 2023 Dec 7;24(24):17222. doi: 10.3390/ijms242417222. Int J Mol Sci. 2023. PMID: 38139052 Free PMC article.
-
The missing link: ARID1B non-truncating variants causing Coffin-Siris syndrome due to protein aggregation.Hum Genet. 2024 Aug;143(8):965-978. doi: 10.1007/s00439-024-02688-9. Epub 2024 Jul 19. Hum Genet. 2024. PMID: 39028335 Free PMC article.
-
Effects of Cycloastragenol on Alzheimer's Disease in Rats by Reducing Oxidative Stress, Inflammation, and Apoptosis.Curr Alzheimer Res. 2024;21(2):141-154. doi: 10.2174/0115672050315334240508162754. Curr Alzheimer Res. 2024. PMID: 38766828
References
-
- Kurplus M., McCammon J.A. Dynamics of proteins: Elements and function. Annu. Rev. Biochem. 1983;52:263–300. - PubMed
-
- Depta L., Whitmarsh-Everiss T., Laraia L. Structure, function and small molecule modulation of intracellular sterol transport proteins. Bioorganic Med. Chem. 2022;68:116856. - PubMed
-
- Kumar R. Role of naturally occurring osmolytes in protein folding and stability. Arch. Biochem. Biophys. 2009;491:1–6. - PubMed
-
- Dobson C.M. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

