Dishevelled 2 regulates cancer cell proliferation and T cell mediated immunity in HER2-positive breast cancer
- PMID: 36809986
- PMCID: PMC9942370
- DOI: 10.1186/s12885-023-10647-2
Dishevelled 2 regulates cancer cell proliferation and T cell mediated immunity in HER2-positive breast cancer
Abstract
Background: Dishevelled paralogs (DVL1, 2, 3) are key mediators of Wnt pathway playing a role in constitutive oncogenic signaling influencing the tumor microenvironment. While previous studies showed correlation of β-catenin with T cell gene expression, little is known about the role of DVL2 in modulating tumor immunity. This study aimed to uncover the novel interaction between DVL2 and HER2-positive (HER2+) breast cancer (BC) in regulating tumor immunity and disease progression.
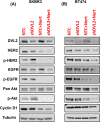
Methods: DVL2 loss of function studies were performed with or without a clinically approved HER2 inhibitor, Neratinib in two different HER2+ BC cell lines. We analyzed RNA (RT-qPCR) and protein (western blot) expression of classic Wnt markers and performed cell proliferation and cell cycle analyses by live cell imaging and flow cytometry, respectively. A pilot study in 24 HER2+ BC patients was performed to dissect the role of DVL2 in tumor immunity. Retrospective chart review on patient records and banked tissue histology were performed. Data were analyzed in SPSS (version 25) and GraphPad Prism (version 7) at a significance p < 0.05.
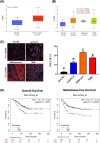
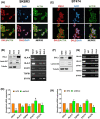
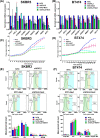
Results: DVL2 regulates the transcription of immune modulatory genes involved in antigen presentation and T cell maintenance. DVL2 loss of function down regulated mRNA expression of Wnt target genes involved in cell proliferation, migration, invasion in HER2+ BC cell lines (±Neratinib). Similarly, live cell proliferation and cell cycle analyses reveal that DVL2 knockdown (±Neratinib) resulted in reduced proliferation, higher growth arrest (G1), limited mitosis (G2/M) compared to non-targeted control in one of the two cell lines used. Analyses on patient tissues who received neoadjuvant chemotherapy (n = 14) further demonstrate that higher DVL2 expression at baseline biopsy pose a significant negative correlation with % CD8α levels (r = - 0.67, p < 0.05) while have a positive correlation with NLR (r = 0.58, p < 0.05), where high NLR denotes worse cancer prognosis. These results from our pilot study reveal interesting roles of DVL2 proteins in regulating tumor immune microenvironment and clinical predictors of survival in HER2+ BC.
Conclusion: Our study demonstrates potential immune regulatory role of DVL2 proteins in HER2+ BC. More in-depth mechanistic studies of DVL paralogs and their influence on anti-tumor immunity may provide insight into DVLs as potential therapeutic targets benefiting BC patients.
Keywords: Breast cancer; Dishevelled; HER2; Tumor infiltrating lymphocytes; Wnt signaling.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Dishevelled segment polarity protein 2 promotes gastric cancer progression through Wnt/β-catenin pathway.Tissue Cell. 2023 Jun;82:102119. doi: 10.1016/j.tice.2023.102119. Epub 2023 May 24. Tissue Cell. 2023. PMID: 37257286
-
Differential mediation of the Wnt canonical pathway by mammalian Dishevelleds-1, -2, and -3.Cell Signal. 2008 Feb;20(2):443-52. doi: 10.1016/j.cellsig.2007.11.005. Epub 2007 Nov 17. Cell Signal. 2008. PMID: 18093802 Free PMC article.
-
CYP2E1 plays a suppressive role in hepatocellular carcinoma by regulating Wnt/Dvl2/β-catenin signaling.J Transl Med. 2022 May 4;20(1):194. doi: 10.1186/s12967-022-03396-6. J Transl Med. 2022. PMID: 35509083 Free PMC article.
-
Dishevelled: A masterful conductor of complex Wnt signals.Cell Signal. 2018 Jul;47:52-64. doi: 10.1016/j.cellsig.2018.03.004. Epub 2018 Mar 17. Cell Signal. 2018. PMID: 29559363 Free PMC article. Review.
-
Ki67 and lymphocytes in the pretherapeutic core biopsy of primary invasive breast cancer: positive markers of therapy response prediction and superior survival.Horm Mol Biol Clin Investig. 2017 Sep 22;32(2):/j/hmbci.2017.32.issue-2/hmbci-2017-0022/hmbci-2017-0022.xml. doi: 10.1515/hmbci-2017-0022. Horm Mol Biol Clin Investig. 2017. PMID: 28937963 Review.
Cited by
-
Automated Detection and Scoring of Tumor-Infiltrating Lymphocytes in Breast Cancer Histopathology Slides.Cancers (Basel). 2023 Jul 15;15(14):3635. doi: 10.3390/cancers15143635. Cancers (Basel). 2023. PMID: 37509295 Free PMC article.
-
Dishevelled localization and function are differentially regulated by structurally distinct sterols.bioRxiv [Preprint]. 2024 May 15:2024.05.14.593701. doi: 10.1101/2024.05.14.593701. bioRxiv. 2024. PMID: 38798572 Free PMC article. Preprint.
References
-
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. - DOI - PMC - PubMed
-
- Carbognin L, Pilotto S, Nortilli R, Brunelli M, Nottegar A, Sperduti I, Giannarelli D, Bria E, Tortora G. Predictive and prognostic role of tumor-infiltrating lymphocytes for early breast cancer according to disease subtypes: sensitivity analysis of randomized trials in adjuvant and neoadjuvant setting. Oncologist. 2016;21(3):283–291. doi: 10.1634/theoncologist.2015-0307. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

