Water channel protein AQP1 in cytoplasm is a critical factor in breast cancer local invasion
- PMID: 36803413
- PMCID: PMC9940370
- DOI: 10.1186/s13046-023-02616-1
Water channel protein AQP1 in cytoplasm is a critical factor in breast cancer local invasion
Abstract
Background: Metastasis of breast cancer grows from the local invasion to the distant colonization. Blocking the local invasion step would be promising for breast cancer treatment. Our present study demonstrated AQP1 was a crucial target in breast cancer local invasion.
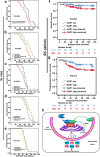
Methods: Mass spectrometry combined with bioinformatics analysis was used to identify AQP1 associated proteins ANXA2 and Rab1b. Co-immunoprecipitation, immunofluorescence assays and cell functional experiments were carried out to define the relationship among AQP1, ANXA2 and Rab1b and their re-localization in breast cancer cells. The Cox proportional hazards regression model was performed toward the identification of relevant prognostic factors. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test.
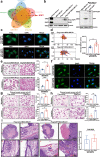
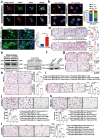
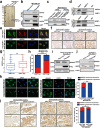
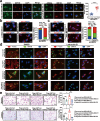
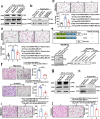
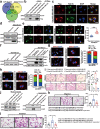
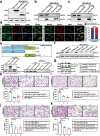
Results: Here, we show that the cytoplasmic water channel protein AQP1, a crucial target in breast cancer local invasion, recruited ANXA2 from the cellular membrane to the Golgi apparatus, promoted Golgi apparatus extension, and induced breast cancer cell migration and invasion. In addition, cytoplasmic AQP1 recruited cytosolic free Rab1b to the Golgi apparatus to form a ternary complex containing AQP1, ANXA2, and Rab1b, which induced cellular secretion of the pro-metastatic proteins ICAM1 and CTSS. Cellular secretion of ICAM1 and CTSS led to the migration and invasion of breast cancer cells. Both in vivo assay and clinical analysis data confirmed above results.
Conclusions: Our findings suggested a novel mechanism for AQP1-induced breast cancer local invasion. Therefore, targeting AQP1 offers promises in breast cancer treatment.
Keywords: AQP1; Breast cancer; Metastasis.
© 2023. The Author(s).
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures
Similar articles
-
Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients.Oncotarget. 2016 Feb 16;7(7):8143-54. doi: 10.18632/oncotarget.6994. Oncotarget. 2016. PMID: 26812884 Free PMC article.
-
[Cytoplasmic expression of aquaporin-1 in breast cancer cells and its relationship with clinicopathological characteristics and prognosis].Zhonghua Zhong Liu Za Zhi. 2013 Dec;35(12):904-9. Zhonghua Zhong Liu Za Zhi. 2013. PMID: 24506959 Chinese.
-
Decreased miR-320 expression is associated with breast cancer progression, cell migration, and invasiveness via targeting Aquaporin 1.Acta Biochim Biophys Sin (Shanghai). 2018 May 1;50(5):473-480. doi: 10.1093/abbs/gmy023. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 29538612
-
The first discovered water channel protein, later called aquaporin 1: molecular characteristics, functions and medical implications.Mol Aspects Med. 2012 Oct-Dec;33(5-6):518-34. doi: 10.1016/j.mam.2012.06.001. Epub 2012 Jun 15. Mol Aspects Med. 2012. PMID: 22705445 Review.
-
Role of Aquaporin 1 Signalling in Cancer Development and Progression.Int J Mol Sci. 2017 Jan 29;18(2):299. doi: 10.3390/ijms18020299. Int J Mol Sci. 2017. PMID: 28146084 Free PMC article. Review.
Cited by
-
Mime: A flexible machine-learning framework to construct and visualize models for clinical characteristics prediction and feature selection.Comput Struct Biotechnol J. 2024 Jun 29;23:2798-2810. doi: 10.1016/j.csbj.2024.06.035. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39055398 Free PMC article.
-
Leptin Promotes Vasculogenic Mimicry in Breast Cancer Cells by Regulating Aquaporin-1.Int J Mol Sci. 2024 May 10;25(10):5215. doi: 10.3390/ijms25105215. Int J Mol Sci. 2024. PMID: 38791252 Free PMC article.
-
The Involvement of Peroxiporins and Antioxidant Transcription Factors in Breast Cancer Therapy Resistance.Cancers (Basel). 2023 Dec 8;15(24):5747. doi: 10.3390/cancers15245747. Cancers (Basel). 2023. PMID: 38136293 Free PMC article. Review.
-
SERPINB5 is a prognostic biomarker and promotes proliferation, metastasis and epithelial-mesenchymal transition (EMT) in lung adenocarcinoma.Thorac Cancer. 2023 Aug;14(23):2275-2287. doi: 10.1111/1759-7714.15013. Epub 2023 Jul 9. Thorac Cancer. 2023. PMID: 37424293 Free PMC article.
-
Characterization of macrophages in head and neck squamous cell carcinoma and development of MRG-based risk signature.Sci Rep. 2024 Apr 30;14(1):9914. doi: 10.1038/s41598-024-60516-6. Sci Rep. 2024. PMID: 38688945 Free PMC article.
References
-
- Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2006;168:1345–1353. doi: 10.2353/ajpath.2006.050596. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

