This is a preprint.
Late-life isoleucine restriction promotes physiological and molecular signatures of healthy aging
- PMID: 36798157
- PMCID: PMC9934591
- DOI: 10.1101/2023.02.06.527311
Late-life isoleucine restriction promotes physiological and molecular signatures of healthy aging
Update in
-
Late-life protein or isoleucine restriction impacts physiological and molecular signatures of aging.Nat Aging. 2024 Dec;4(12):1760-1771. doi: 10.1038/s43587-024-00744-7. Epub 2024 Nov 27. Nat Aging. 2024. PMID: 39604703
Abstract
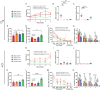
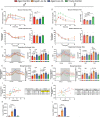
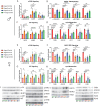
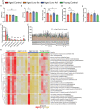
In defiance of the paradigm that calories from all sources are equivalent, we and others have shown that dietary protein is a dominant regulator of healthy aging. The restriction of protein or the branched-chain amino acid isoleucine promotes healthspan and extends lifespan when initiated in young or adult mice. However, many interventions are less efficacious or even deleterious when initiated in aged animals. Here, we investigate the physiological, metabolic, and molecular consequences of consuming a diet with a 67% reduction of all amino acids (Low AA), or of isoleucine alone (Low Ile), in male and female C57BL/6J.Nia mice starting at 20 months of age. We find that both diet regimens effectively reduce adiposity and improve glucose tolerance, which were benefits that were not mediated by reduced calorie intake. Both diets improve specific aspects of frailty, slow multiple molecular indicators of aging rate, and rejuvenate the aging heart and liver at the molecular level. These results demonstrate that Low AA and Low Ile diets can drive youthful physiological and molecular signatures, and support the possibility that these dietary interventions could help to promote healthy aging in older adults.
Keywords: BCAA; aging; dietary protein; dietary restriction; isoleucine; metabolic health; protein restriction.
Conflict of interest statement
D.W.L has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The remaining authors declare no competing interests.
Figures

Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Late-life protein or isoleucine restriction impacts physiological and molecular signatures of aging.Nat Aging. 2024 Dec;4(12):1760-1771. doi: 10.1038/s43587-024-00744-7. Epub 2024 Nov 27. Nat Aging. 2024. PMID: 39604703
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
References
-
- Bellantuono I., de Cabo R., Ehninger D., Di Germanio C., Lawrie A., Miller J., Mitchell S.J., Navas-Enamorado I., Potter P.K., Tchkonia T., et al. (2020). A toolbox for the longitudinal assessment of healthspan in aging mice. Nature protocols 15, 540–574. 10.1038/s41596-019-0256-1. - DOI - PMC - PubMed
Publication types
Grants and funding
- R56 AG056771/AG/NIA NIH HHS/United States
- F31 AG066311/AG/NIA NIH HHS/United States
- I01 BX004031/BX/BLRD VA/United States
- F31 AG082504/AG/NIA NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- F32 AG077916/AG/NIA NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- R01 DK125859/DK/NIDDK NIH HHS/United States
- U01 AG081482/AG/NIA NIH HHS/United States
- F31 AG081115/AG/NIA NIH HHS/United States
- R01 DK133479/DK/NIDDK NIH HHS/United States
- IS1 BX005524/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- F99 AG083290/AG/NIA NIH HHS/United States
- R01 AG084156/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
