Advances in NK cell therapy for brain tumors
- PMID: 36792722
- PMCID: PMC9932101
- DOI: 10.1038/s41698-023-00356-1
Advances in NK cell therapy for brain tumors
Abstract
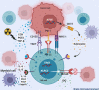
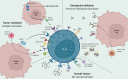
Despite advances in treatment regimens that comprise surgery, chemotherapy, and radiation, outcome of many brain tumors remains dismal, more so when they recur. The proximity of brain tumors to delicate neural structures often precludes complete surgical resection. Toxicity and long-term side effects of systemic therapy remain a concern. Novel therapies are warranted. The field of NK cell-based cancer therapy has grown exponentially and currently constitutes a major area of immunotherapy innovation. This provides a new avenue for the treatment of cancerous lesions in the brain. In this review, we explore the mechanisms by which the brain tumor microenvironment suppresses NK cell mediated tumor control, and the methods being used to create NK cell products that subvert immune suppression. We discuss the pre-clinical studies evaluating NK cell-based immunotherapies that target several neuro-malignancies and highlight advances in molecular imaging of NK cells that allow monitoring of NK cell-based therapeutics. We review current and ongoing NK cell based clinical trials in neuro-oncology.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Exploring the NK cell platform for cancer immunotherapy.Nat Rev Clin Oncol. 2021 Feb;18(2):85-100. doi: 10.1038/s41571-020-0426-7. Epub 2020 Sep 15. Nat Rev Clin Oncol. 2021. PMID: 32934330 Free PMC article. Review.
-
Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy?Front Immunol. 2020 Feb 21;11:275. doi: 10.3389/fimmu.2020.00275. eCollection 2020. Front Immunol. 2020. PMID: 32153582 Free PMC article. Review.
-
Recent Advances to Augment NK Cell Cancer Immunotherapy Using Nanoparticles.Pharmaceutics. 2021 Apr 9;13(4):525. doi: 10.3390/pharmaceutics13040525. Pharmaceutics. 2021. PMID: 33918941 Free PMC article. Review.
-
Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity.Front Immunol. 2015 Nov 30;6:605. doi: 10.3389/fimmu.2015.00605. eCollection 2015. Front Immunol. 2015. PMID: 26648934 Free PMC article. Review.
-
Next Generation Natural Killer Cells for Cancer Immunotherapy.Front Immunol. 2022 Jun 2;13:886429. doi: 10.3389/fimmu.2022.886429. eCollection 2022. Front Immunol. 2022. PMID: 35720306 Free PMC article. Review.
Cited by
-
Recent Advancements in Biomaterials for Chimeric Antigen Receptor T Cell Immunotherapy.Biomater Res. 2024 Jul 15;28:0045. doi: 10.34133/bmr.0045. eCollection 2024. Biomater Res. 2024. PMID: 39011521 Free PMC article. Review.
-
Immune cell infiltration and inflammatory landscape in primary brain tumours.J Transl Med. 2024 May 30;22(1):521. doi: 10.1186/s12967-024-05309-1. J Transl Med. 2024. PMID: 38816839 Free PMC article.
-
Feeder-free differentiation of human iPSCs into natural killer cells with cytotoxic potential against malignant brain rhabdoid tumor cells.Bioact Mater. 2024 Mar 8;36:301-316. doi: 10.1016/j.bioactmat.2024.02.031. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38496035 Free PMC article.
-
Chimeric antigen receptors in the brain: Can we tackle glioblastoma with engineered NK cells?Neuro Oncol. 2023 Nov 2;25(11):2072-2073. doi: 10.1093/neuonc/noad131. Neuro Oncol. 2023. PMID: 37522296 Free PMC article. No abstract available.
-
Targeting Innate Immunity in Glioma Therapy.Int J Mol Sci. 2024 Jan 12;25(2):947. doi: 10.3390/ijms25020947. Int J Mol Sci. 2024. PMID: 38256021 Free PMC article. Review.
References
-
- Tallman, M. M., Zalenski, A. A. & Venere, M. In Gliomas (ed. Debinski, W.) (2021). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

