The PYK2 inhibitor PF-562271 enhances the effect of temozolomide on tumor growth in a C57Bl/6-Gl261 mouse glioma model
- PMID: 36790653
- PMCID: PMC9992029
- DOI: 10.1007/s11060-023-04260-3
The PYK2 inhibitor PF-562271 enhances the effect of temozolomide on tumor growth in a C57Bl/6-Gl261 mouse glioma model
Abstract
Background: The development of resistance to temozolomide (TMZ), a standard chemotherapeutic, limits the effective treatment of glioblastoma (GBM). Focal adhesion kinase (FAK) and proline rich tyrosine kinase 2 (Pyk2) regulate proliferation and invasion of GBM cells. We found that TMZ activates FAK and Pyk2 signaling in GBM. We hypothesized that pharmacological inhibitors of Pyk2/FAK together with TMZ can enhance the inhibitory effect of TMZ on tumor growth and dispersal and improve the treatment outcome.
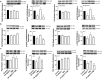
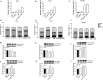
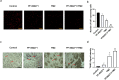
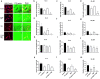
Methods: Primary human GBM cell cultures and a C57Bl/6-GL261 mouse glioma implantation model were used. Pyk2 (Tyr579/580) and FAK (Tyr925) phosphorylation was analyzed by western blotting. Viability, cell cycle, migration, invasion and invadopodia formation were investigated in vitro. Animal survival, tumor size and invasion, TUNEL apoptotic cell death and the Ki67 proliferation index were evaluated in vivo upon treatment with TMZ (50 mg/kg, once/day, orally) and the Pyk2/FAK inhibitor PF-562271 (once/daily, 50 mg/kg, orally) vs. TMZ monotherapy.
Results: In vitro studies revealed significantly reduced viability, cell cycle progression, invasion and invadopodia with TMZ (100 µM) + PF-562271 (16 nM) compared with TMZ alone. In vivo studies demonstrated that combinatorial treatment led to prominent reductions in tumor size and invasive margins, extensive signs of apoptosis and a reduced proliferation index, together with a 15% increase in the survival rate in animals, compared with TMZ monotherapy.
Conclusion: TMZ + PF-562271 eliminates TMZ-related Pyk2/FAK activation in GBM and improves the treatment efficacy.
Keywords: FAK; GBM; PF-562271; Pyk2; Temozolomide.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or nonfinancial interests to disclose.
Figures
Similar articles
-
Pyk2/FAK Signaling Is Upregulated in Recurrent Glioblastoma Tumors in a C57BL/6/GL261 Glioma Implantation Model.Int J Mol Sci. 2023 Aug 30;24(17):13467. doi: 10.3390/ijms241713467. Int J Mol Sci. 2023. PMID: 37686276 Free PMC article.
-
Microglia Activate Migration of Glioma Cells through a Pyk2 Intracellular Pathway.PLoS One. 2015 Jun 22;10(6):e0131059. doi: 10.1371/journal.pone.0131059. eCollection 2015. PLoS One. 2015. PMID: 26098895 Free PMC article.
-
Induction of proline-rich tyrosine kinase 2 activation-mediated C6 glioma cell invasion after anti-vascular endothelial growth factor therapy.J Transl Med. 2014 May 27;12:148. doi: 10.1186/1479-5876-12-148. J Transl Med. 2014. PMID: 24884636 Free PMC article.
-
Riluzole enhances the antitumor effects of temozolomide via suppression of MGMT expression in glioblastoma.J Neurosurg. 2020 Mar 13;134(3):701-710. doi: 10.3171/2019.12.JNS192682. Print 2021 Mar 1. J Neurosurg. 2020. PMID: 32168477
-
Cordycepin Augments the Chemosensitivity of Human Glioma Cells to Temozolomide by Activating AMPK and Inhibiting the AKT Signaling Pathway.Mol Pharm. 2018 Nov 5;15(11):4912-4925. doi: 10.1021/acs.molpharmaceut.8b00551. Epub 2018 Oct 17. Mol Pharm. 2018. PMID: 30336060
Cited by
-
Pyk2/FAK Signaling Is Upregulated in Recurrent Glioblastoma Tumors in a C57BL/6/GL261 Glioma Implantation Model.Int J Mol Sci. 2023 Aug 30;24(17):13467. doi: 10.3390/ijms241713467. Int J Mol Sci. 2023. PMID: 37686276 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

